
Contributed
Multi-doped electrocatalysts for efficient hydrazine oxidation
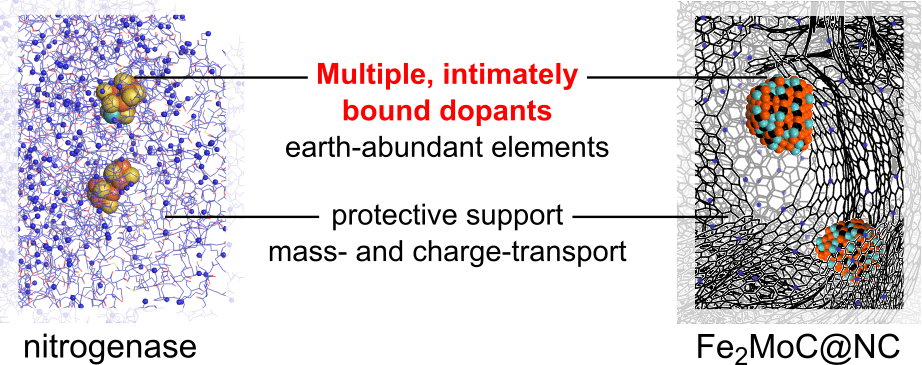
We report an efficient electrocatalyst for the oxidation of hydrazine, a promising fuel for fuel cells and an important analyte for health and environmental monitoring.[1] To design this material, we took a biomimetic approach. Namely, we emulated natural nitrogen-cycle enzymes, focusing on designing a cooperative, multi-doped active site. The catalytic oxidation occurs on Fe2MoC nanoparticles and on edge-positioned nitrogen dopants, all well-dispersed on a hierarchically porous, graphitic carbon matrix that provides active site exposure to mass-transfer and charge flow. The new catalyst is the first carbide known to catalyse hydrazine electro-oxidation. It operates at the most negative onset potentials reported for carbon-based hydrazine oxidation catalysts at pH 14 (0.28 V vs. RHE), and has good-to-excellent activity at pH values down to 0. It shows high faradaic efficiency for oxidation to N2 (3.6 e–/N2H4), and is perfectly stable for at least 2000 cycles. Building on this discovery, we investigate how hydrazine oxidation activity is determined by (1) doping of the carbide nanoparticles, and (2) rational nanostructuring of the carbon matrix.
[1] K. Ojha, E. M. Farber, T. Burshtein, D. Eisenberg, Angew. Chem. Int. Ed. 2018, doi: 10.1002/anie.201810960.
Powered by Eventact EMS