
Tunneling and aromaticity tuning in fulvalene: A computational study
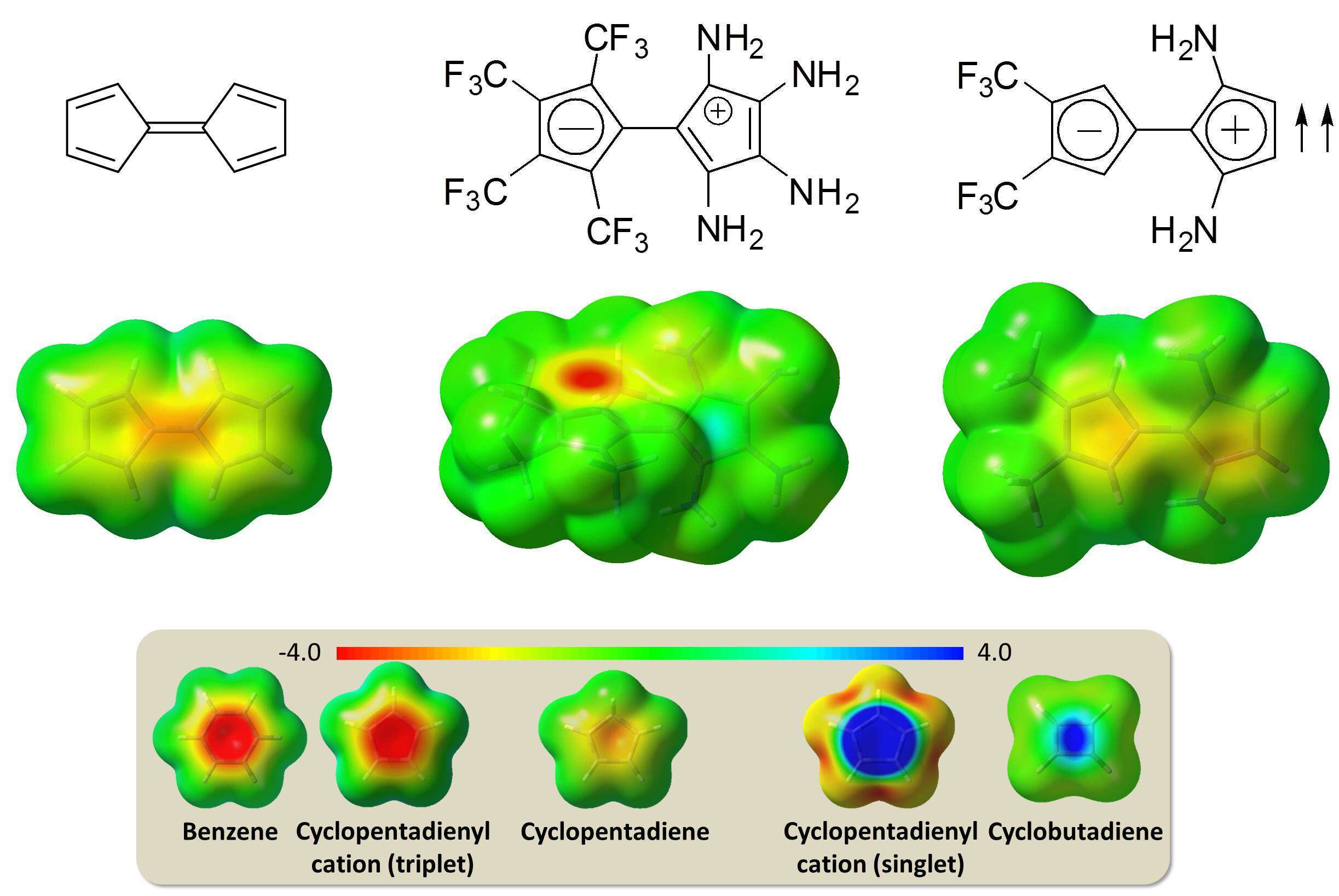
Fulvalenes are a family of molecules built from two fully-conjugated rings, connected to one another by an exocyclic double bond. Pentafulvalene, having two five-membered rings that are each one electron short of a 6π-electron aromatic system, is non-aromatic and has no dipole moment. We investigated computationally various derivatives of pentafulvalene and found that placing strong electron withdrawing groups on one ring and strong electron donating groups on the second ring can induce significant charge transfer between the rings, and stabilize a zwitterionic structure. This produces one aromatic and one antiaromatic ring in the same molecule. We also found that such derivatives can undergo π-system shifting in the antiaromatic ring via quantum mechanical tunneling even at temperatures very close to 0 K. In addition, triplets of such pentafulvalene derivatives exhibit a charge transfer diradical structure, as the triplet is localized on the positively charged ring. This produces one ring with Hückel 4n+2 aromaticity, and a second ring that is a 4π-electron triplet system and is aromatic according to Baird’s rule. However, in most of the derivatives tested, the Jahn-Teller effect wins over Hund’s rule and the singlet is more stable than the triplet. Even so, a right arrangement of the substituents can stabilize the triplet, and thus produce a novel neutral pentafulvalene having two aromatic rings in its ground state.
Powered by Eventact EMS