
Sunlight-assisted synthesis of amides and peptides
2Department of Chemical Sciences, Ariel University, Ariel, Israel
The sun delivers a constant flux of free energy to earth in the form of sun-light that may be harnessed to perform diverse chemical reactions.1 We have developed a new synthetic methodology for photo-assisted and photo-induced synthesis of organic compounds.2 The process is based on light activation of amine-alkylhalide charge-transfer complexes, for example the charge-transfer complex between DABCO and CCl4 [DABCO•••CCl4].3 Upon light excitation of the complex, halogen is transferred from the alkylhalide to the amine, forming halonium active species. These may be used for the performance of different chemical reactions, such as dealkylative amidation using tertiary and secondary amines and dehydrated amidation with primary amines.2,4 Using a similar [DMAP•••CCl4] charge-transfer complex it is now possible to form amide bond between free carboxylic acids and primary amines including for peptide synthesis, Scheme 1. Excellent isolated yields of both amides and peptides are obtained within 12-26 h, without epimerization of stereogenic centers.4 A major advantage is that, unlike most photochemical reactions, the weak light absorption of sun-light by the charge-transfer complexes allows simple scale-up, avoiding elaborate expensive equipment.
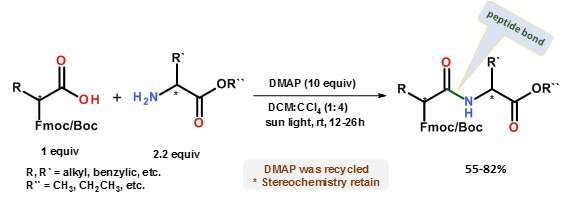
Scheme 1. Schematic representation of the synthesis of peptides using various amino acids via an in situ generated DMAP•••CCl4 charge transfer complex.
References
- E. Robinowitch, Govindjee. Photosynthesis, John Wiley & Sons, Inc., New York, 2014.
- Irit Cohen, Abhaya K. Mishra, Galit Parvari, Rachel Edrei, Mauricio Dantus, Yoav Eichen, Alex M. Szpilman Chem. Commun. 2017, 53, 10128.
- Abhaya K. Mishra, Galit Parvari, Irit Cohen, Natalia Fridman, Yoav Eichen, Alex M. Szpilman J. Coord. Chem. 2018, 71, 2082.
- Abhaya K. Mishra, Galit Parvari, Yoav Eichen, Alex M. Szpilman Manuscript in Preparation.
Powered by Eventact EMS