
Flash
The mechanism of catalytic hydrogen evolution on M0 nanoparticles suspended in aqueous solutions
2Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
3Department of Chemistry, Nuclear Research Center Negev, Beer-Sheva, Israel
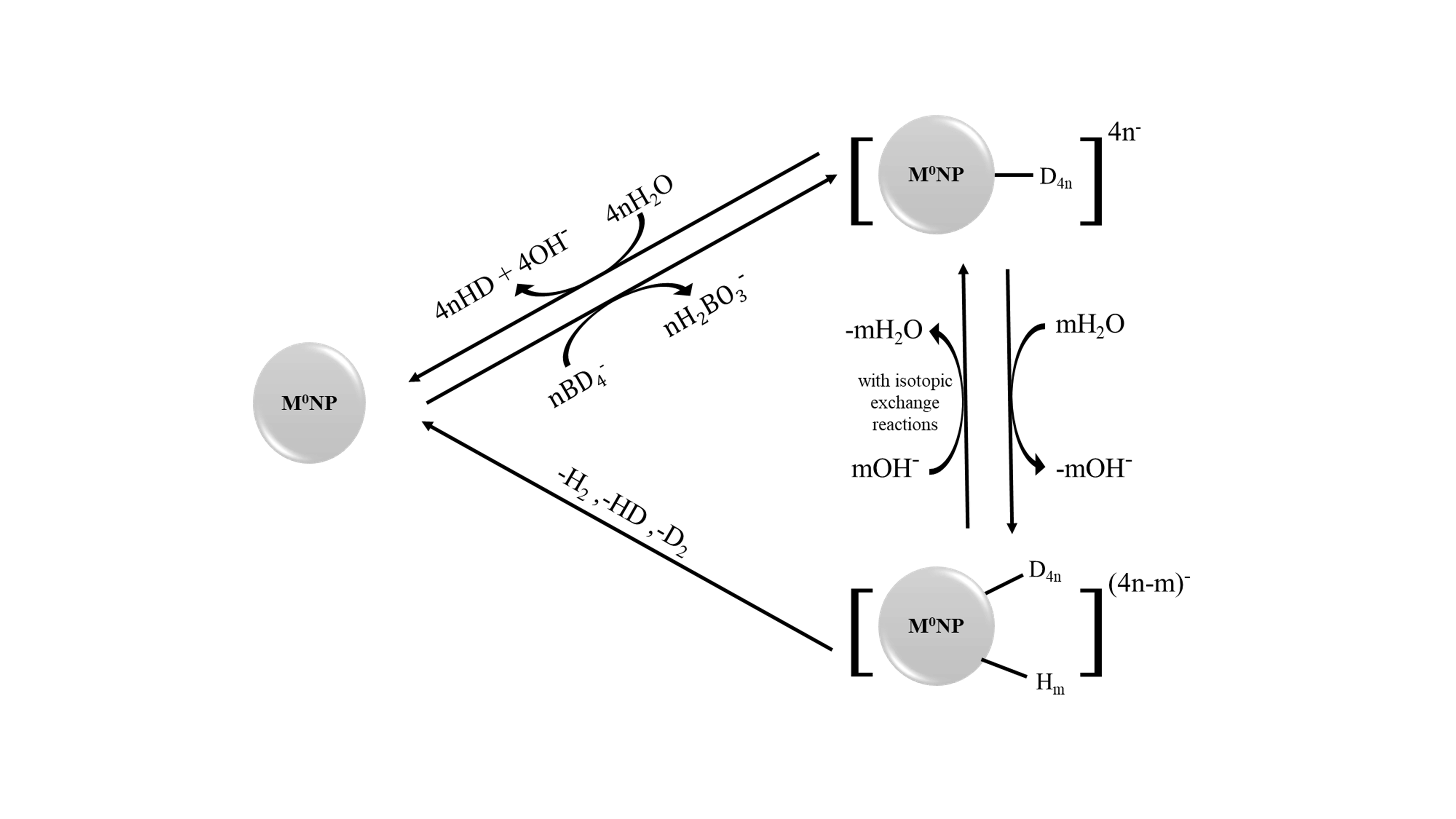
Nowadays, metals are an integral part of the chemical industry. In particular, noble metals are extensively employed in industry, agriculture, jewelry and in the medicinal world. A variety of industrial catalytic reactions involve extensive usage of metals, especially in solid-state catalysis. Often, poisoning of metal surfaces occurs during catalytic reactions under extreme conditions results in a widespread impact on environmental pollution. One of the most studied resolutions for this environmental problem is catalysis based on nanoparticles (NPs) and in particular on metal NPs in aqueous solutions. NPs are known for their high catalytic efficiency in mild conditions, and they are extensively investigated due to their surface to volume ratio. The nature of H-atoms adsorbed on M0-nanoparticles is of major importance in many catalyzed reduction processes. Thus, we have chosen to use isotope labeling (reduction with NaBD4), and successfully determined that hydrogen evolution from the transient {(M0-NP)-Hn}n- proceeds mainly via the Heyrovsky mechanism when n is large (i.e., the hydrogens behave as hydrides) but mainly via the Tafel mechanism when n is small (i.e., the hydrogens behave as atoms)1. Additionally, the relative contributions of the two mechanisms differ considerably for M=Au and Ag. The results are analogous to those recently reported for the M0-NP-catalyzed de-halogenation processes.2
* This study was supported by the Pazy Foundation
- Sermiagin, A., Meyerstein, D., Bar-Ziv, R. and Zidki, T., Angew. Chemie Int. Ed. 1–5 (2018). doi:10.1002/anie.201809302
- Adhikary, J. et al., Appl. Catal. B Environ. 239, 450–462 (2018).
Powered by Eventact EMS