
Reactivity enhancement via steric restriction of the active site in branched catalysts for phosphorylation reactions
Phosphorylation of alcohol groups in peptides, proteins, and steroids plays a crucial role in many physiologically important processes. Moreover, phosphate derivatives are prepared in order to increase the solubility of alcohols, e.g. in prodrugs. Accordingly, the development of synthetic methods for the efficient, cost-effective and scalable phosphorylation of alcohol derivatives is an important goal.
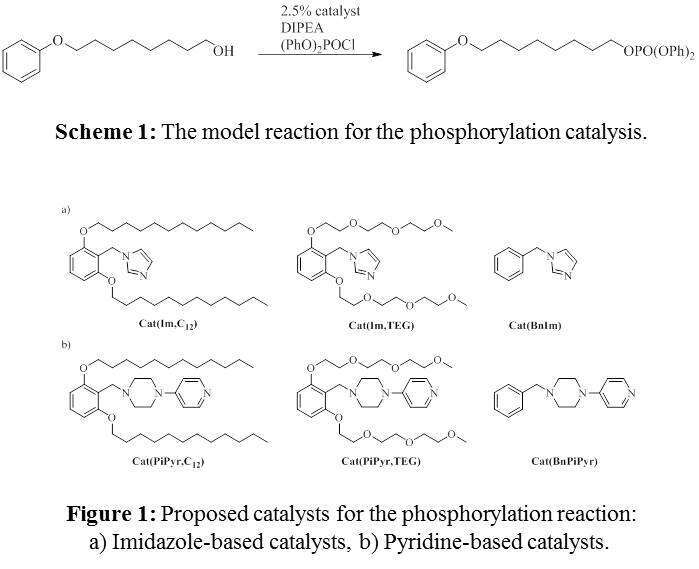
Different Lewis bases can be used to catalyze phosphorylation of alcohols, the most common are pyridine-based, as well as imidazole-based compounds. In the past, it was observed in our group that, sterically restricted imidazole-based catalysts (Figure 1a) are more reactive than their non-restricted analogue for the model phosphorylation reaction (Scheme 1). In an attempt to exploit this approach, we designed and synthesized catalysts incorporating a pyridine-based catalytic unit (Figure 1b), and examined their reactivity in the model reaction.
Both types of catalytic units demonstrated the same trend: a major increase in the reactivity was obtained for the catalysts with oligo(ethylene glycol) arms, while for those with aliphatic arms only a minor improvement was observed. The comparison of the restricted catalysts with analogues, lacking the shielding elements, demonstrated that the chosen design indeed enhances the kinetics of the phosphorylation catalytic process. Additional shielded organocatalytic structures which are even more reactive, as well as the catalysis with other alcohol substrates, will be disclosed.
Powered by Eventact EMS