
Contributed
Recycling chelators with a chelating polymer: What indeed happens on the molecular level?
2School of Mechanical Engineering, Faculty of Engineering, Tel Aviv University, Tel Aviv, Israel
As part of the project of developing a “green” and highly feasible soil remediation process, recycling an eco-friendly chelating agent, glycine, using Chelex-100 chelating polymer, was studied. Two model complexes, copper and nickel glycinates, were tested under various conditions, demonstrating very different reactivity towards Chelex-100. An in-depth study led to the discovery of unusual metal-dependent mechanisms of the complex-to-polymer metal transfer. Particularly, nickel transfer proceeds via a dissociative mechanism, whereas copper transfer does not require pre-dissociation of the complexes, and proceeds via the associative ligand-exchange mechanism. Both processes result in the recovery of the used chelator (glycine).
The glycine solution was applied on the spiked soil, then recovered on Chelex-100, and successfully reused, thus demonstrating a proof of the concept. These findings contribute to the science, strategies, and methodology of water/soil purification and chelator recycling areas.
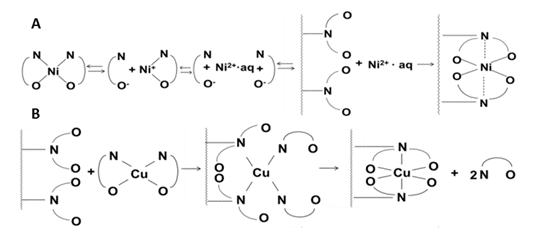
Reference: N. Dolev, Z. Katz, Z. Ludmer, A. Ullmann, N. Brauner, R. Goikhman*. Chemosphere, 215 (2019) 800–806.
Powered by Eventact EMS