
Influence of acids, bases and catalysts on substrate-selectivity of acylation reaction
Acyl-transfer reactions are of the most common transformations in organic synthesis, as well as in Nature. Due to their importance in biochemical and synthetic processes, these reactions have been widely studied. The acylation of alcohols is a common transformation that can be promoted by a variety of catalysts. Among them, donor-substituted pyridine derivatives, such as DMAP, play an important role as nucleophilic catalysts. In spite of the widespread use of the acylation reaction, conducting it in a substrate- or site-selective manner still represents a major challenge.
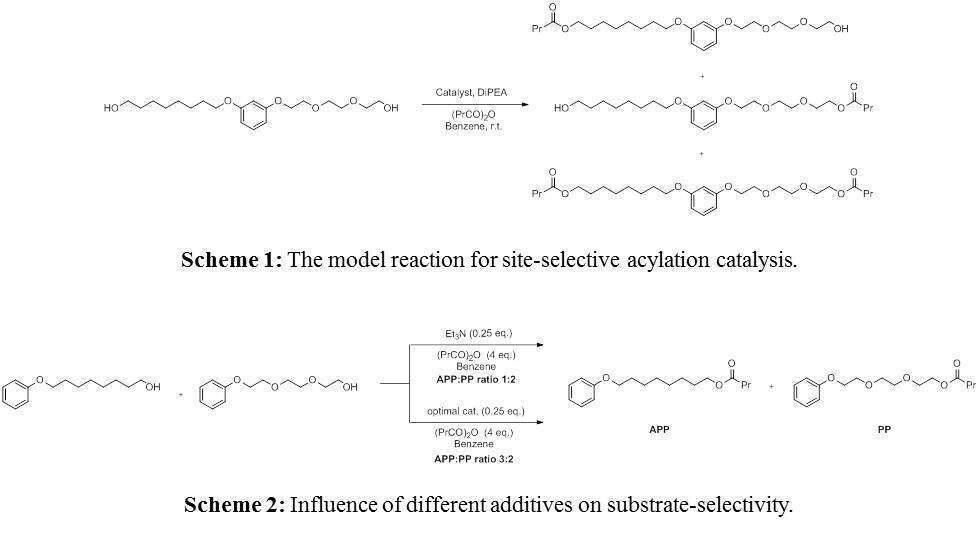
During the development of catalysts for a site-selective acylation reaction (Scheme 1), we found that there is a strong influence of various additives, such as catalysts and bases, which are usually added to the catalytic system, on the substrate- and site-selectivity. Moreover, we discovered that an addition of acidic additive can exert a profound effect on the substrate-selectivity, sometimes neutralizing or overpowering the effects of the other reaction components (Scheme 2). Various consequences of these findings will be presented.
Powered by Eventact EMS