
Copper(II) catalyses the reduction of perchlorate by both formaldehyde and by dihydrogen in aqueous solutions
1
1,2
1,2
3
4
5
2
1
1
6
2,6
1Department of Chemistry, Nuclear Research Center Negev, Beer-Sheva, Israel
2Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
3Department of Chemical Engineering, Sami Shamoon College of Engineering, Beer-Sheva, Israel
4Department of Chemical Engineering, Biotechnology and Materials, Ariel University, Ariel, Israel
5Department of Physics, Ben-Gurion University of the Negev, Beer-Sheva, Israel
6Department of Chemical Sciences, Ariel University, Ariel, Israel
2Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
3Department of Chemical Engineering, Sami Shamoon College of Engineering, Beer-Sheva, Israel
4Department of Chemical Engineering, Biotechnology and Materials, Ariel University, Ariel, Israel
5Department of Physics, Ben-Gurion University of the Negev, Beer-Sheva, Israel
6Department of Chemical Sciences, Ariel University, Ariel, Israel
During an effort to synthesize the trans-III-copper(II)-1,4,8,11-tetramethyl-pyro-phosphonate-1,4,8,11-tetra-aza-cyclo-tetradecane, using only metal perchlorate salts, it was noted that the perchlorate is reduced to chloride. This surprising result may be explained only by the fact that Cu2+(aq) catalyzes the reduction of perchlorate by formaldehyde or by H2. These reactions are slow at room temperature and ambient pressures. DFT calculations support the involvement of the transient CuH+ in the reduction process.
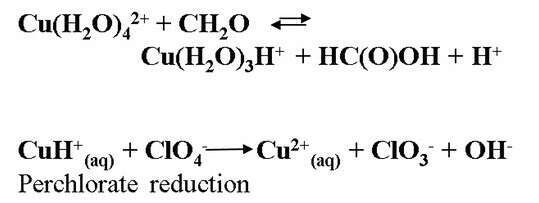
Powered by Eventact EMS