
How Zn2+ ions affect the structure of the neurokinin A (NKA), neurokinin B (NKB) and substance P (SP)?
2Ilse Katz Institute for Nanoscale Science and Technology, Ben-Gurion University of the Negev, Beer-Sheva, Israel
3Department of Chemistry, University of Wrocław, Wrocław, Poland
Neurokinin A (NKA), Neurokinin B (NKB) and Substance P (SP) are the most familiar neuropeptides which are part of large class of molecules that play important biological functions in the brain. Zinc is known to be released from neurons during neuronal activity. It is hypothesized that these neuropeptides have the ability to bind Zn2+ ions. In the current study, we examine the Zn2+-binding sites in NKA, NKB and SP and the effect of Zn2+ ions on their structures.
Our simulations show that in absence of Zn2+ ions, NKA has 2 main conformations: helical (Figure 1a) and random coil conformations. The experiments show that NKA is a random coiled. In presence of Zn2+ ions, both the simulations and the experiment illustrate that NKA show equilibration between α-helix (Figure 1b) and random coiled conformations.
Interestingly, in absence of Zn2+ ions, NKB has slightly helical structure and SP is a random coiled, but in presence of Zn2+ ions, NKB and SP are random coiled. These two peptides conserve the Zn2+-binding site (In NKB: Asp1, Asp4, and in SP: Two O atoms in the carboxylic group of the C-terminal). But, the Zn2+ ion in NKA is hopping between His1 and Asp4 (which is the preferred binding site) to the two O atoms in the carboxylic group of C-terminal. Therefore, we propose that NKB and SP can serve as excellent Zn2+ chelators for inhibition of amyloid aggregation.
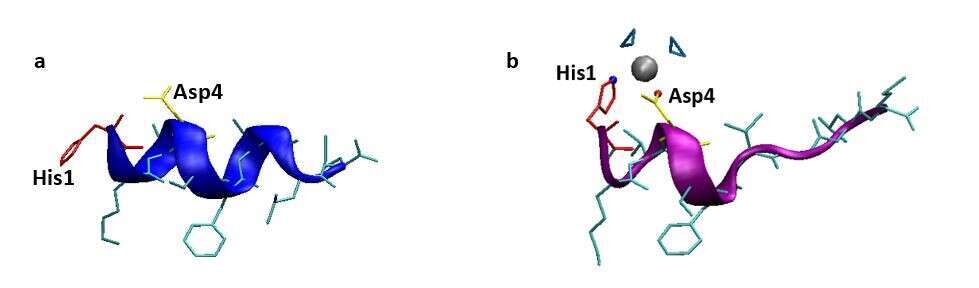
Figure 1: Representative structural models of NKA (sequence: HKTDSFVGLM) in (a) absence of Zn2+ and (b) in presence of Zn2+.
Powered by Eventact EMS