
Insights into new molecular effects in polymorphic Aβ 1-42 dimers
Soluble amyloid β (Aβ) oligomers are known as toxic species that are
associated with the pathology of Alzheimers disease (AD). The smallest
oligomers - Aβ dimers, are considered as building blocks for higher molecular
weight toxic oligomers. Understanding the molecular mechanisms of the
formation of these early-stage Aβ dimers may assist in developing effective
inhibitors for Aβ aggregation.
Using molecular dynamics simulations for two polymorphic Aβ1-42 dimer models,
we illustrate two different molecular effects that govern the formation of Aβ dimers:
(1) “Hydrophobic clustering effect” - a cluster of hydrophobic residues that
induce seeding in Aβ dimer to produce fibril-like Aβ1-42 dimer. (2) “Helix-β-
hairpin-Helix wave effect” - conformational changes of secondary structure in Aβ
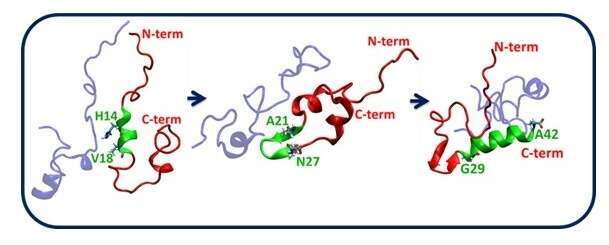
monomer within the disordered Aβ1-42 dimer. The “wave effect” is started from the
N-terminal and is advanced to the C-terminal domain: The N terminal α-helix
(residues H14-V18) induces the formation of β-hairpin structure at the central
domain (residues A21-N27) then the formation of α-helix in the C-terminal domain
(residues G29-A42)(Figure 1).
Figure 1: “Helix-β-hairpin-Helix wave effect” in a disordered Aβ1-42 dimer.
Powered by Eventact EMS