
Electrochemical hydroxylation of arenes catalyzed by a Keggin polyoxometalate with a cobalt(IV) heteroatom
The sustainable, selective direct hydroxylation of arenes, such as benzene to phenol, is an important research challenge. An electrocatalytic transformation using formic acid to oxidize benzene and its halogenated derivatives to selectively yield aryl formates, which are easily hydrolyzed by water to yield the corresponding phenols, is presented (Figure1). The formylation reaction occurs on a Pt anode in the presence of [CoIIIW12O40]5− as a catalyst and lithium formate as an electrolyte via formation of a formyloxyl radical, HOCO•, as the reactive species, which was trapped by a BMPO spin trap and identified by EPR (Figure 2). Hydrogen was formed at the Pt cathode. The sum transformation is ArH+H2O→ArOH+H2. Non‐optimized reaction conditions showed a Faradaic efficiency of 75 % and selective formation of the mono‐oxidized product in a 35 % yield. Decomposition of formic acid into CO2 and H2 is a side‐reaction.
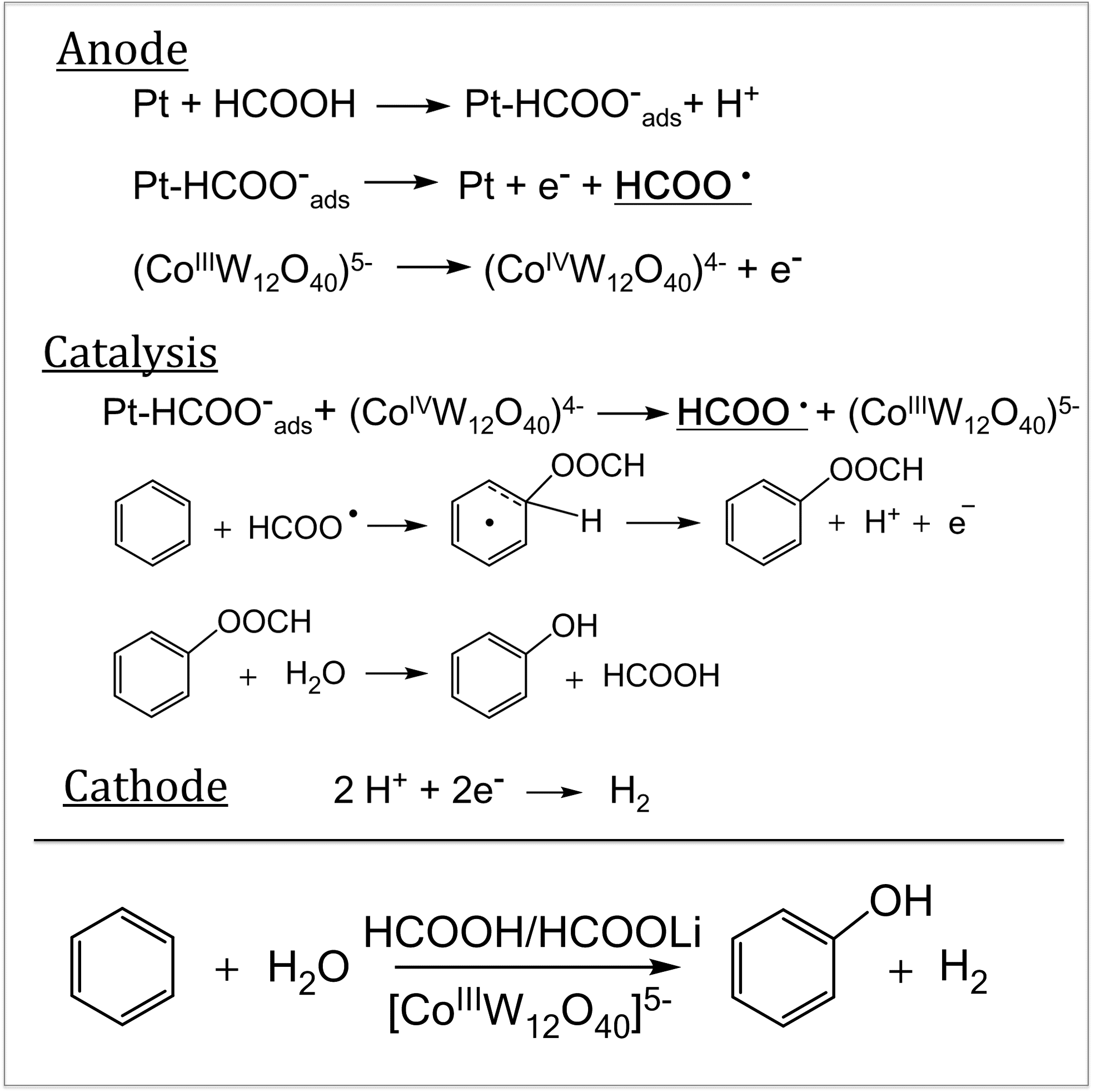
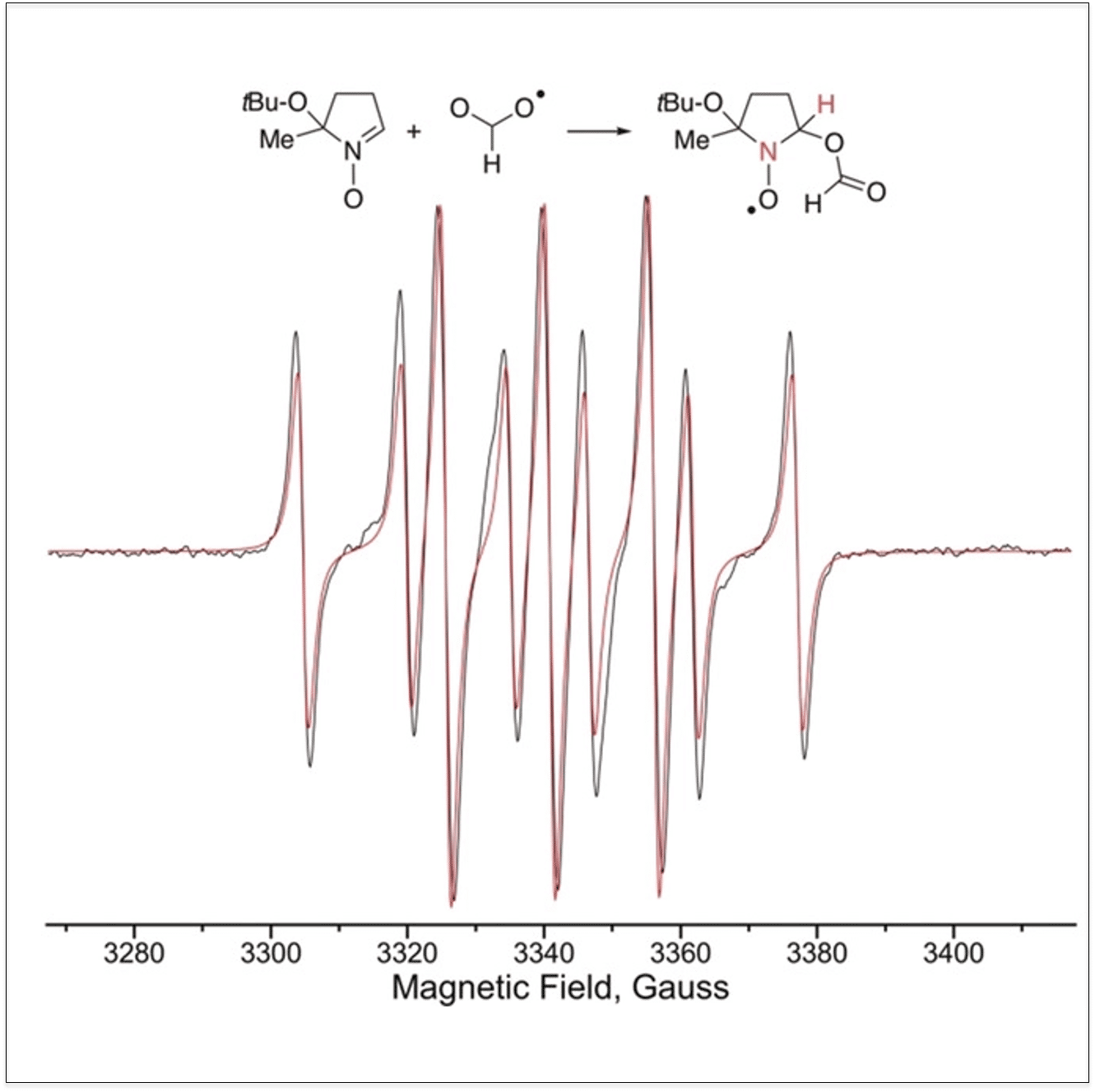
Figure 1:Suggested pathway for benzene oxidation Figure 2: EPR Spectrum of the spin adduct of BMPO and the formyloxy radical. Experimental spectrum (black) and simulated spectrum (red). The hyperfine splitting is due to the N and H atoms shown in red.
Powered by Eventact EMS