
Reactant-induced and particle size-dependent effects of restructuring in heterogeneous catalysis
2Department of Materials Science and Chemical Engineering, Stony Brook University, Stony Brook, NY, USA
3Department of Chemical Engineering and Chemistry, Technical University of Eindhoven, Eindhoven, Netherlands
Some fundamental concepts of heterogeneous catalysis are as of yet not fully explained, yet are of paramount importance in the chemical industry. An example is the concept of structure insensitivity (of which a classical illustration is olefin hydrogenation), which is defined as when the surface-normalized activity of a reaction does not change with catalyst metal particle size [1]. In this work we explore the concept of structure insensitivity and its relation to surface reconstruction in a non-model study on a range of well-defined silica-supported Ni nanoparticular catalysts [2]. By use of state-of-the-art operando micro- and spectroscopic methods, the role of surface restructuring becomes clear in what we term apparently structure insensitive reactions. Furthermore, we show that the reversibility of adsorbate-induced restructuring by ethene decreases with particle size, and that larger particles have a higher C diffusion coefficient. By introducing these new spectroscopic techniques and data analysis methods, and linking them to non-model systems at work, we open up the discussion to several fundamental concepts in heterogeneous catalysts, which may help us to design new or improved solid catalysts for various reactions.
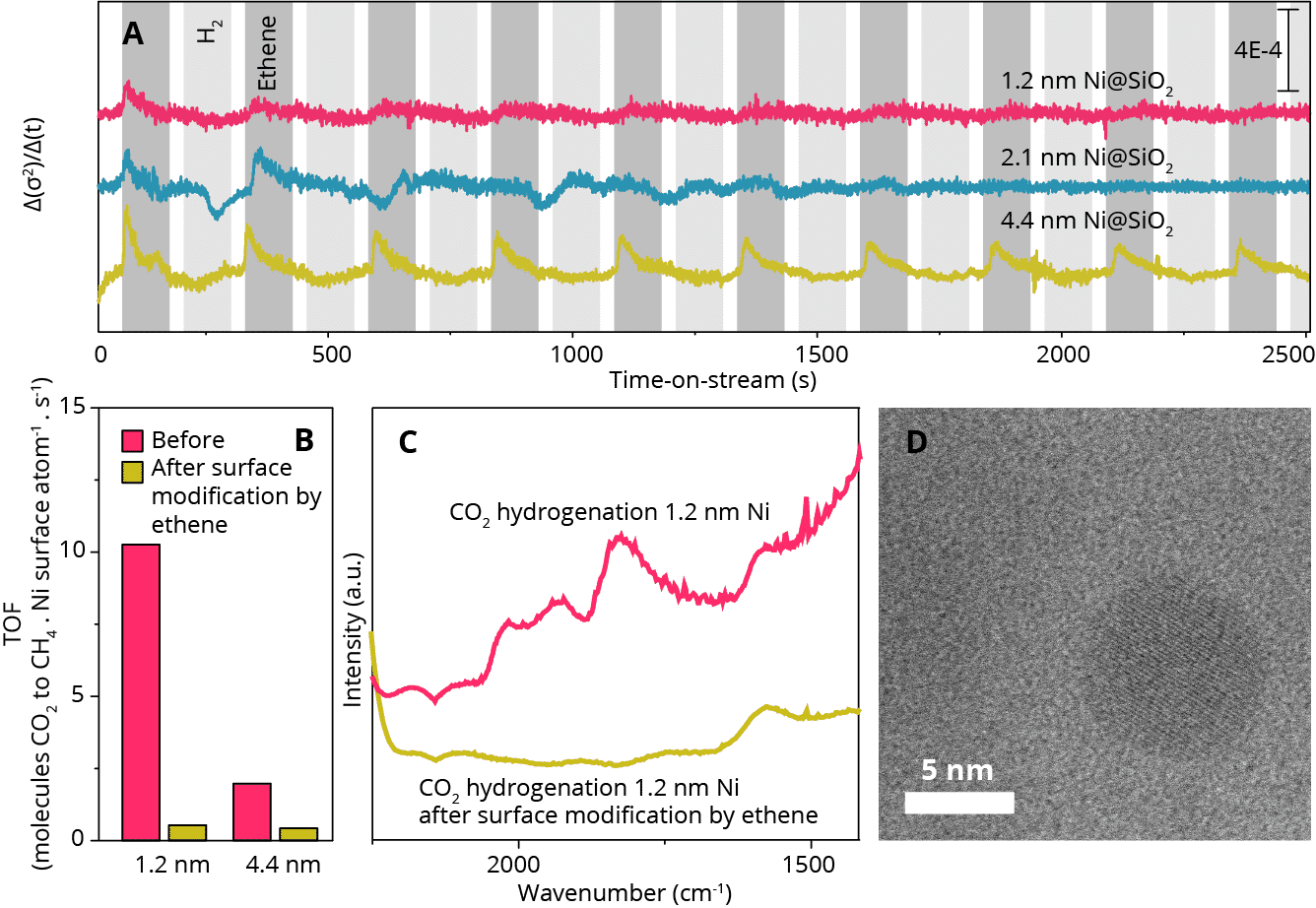
Figure 1: a) The change in Debye-Waller factor against time obtained from individual fits of 70.000 quick-XAS spectra per experiment. b) Turnover frequencies and c) operando FT-IR spectroscopy of CO2 hydrogenation before, and after surface modification. d) in-situ STEM image of Ni nanoparticle at 400 °C, in 1 bar of ethylene.
References
[1] Somorjai, G. A.; Catal. Lett. 7, 169–182 (1990).
[2] Vogt, C., Groeneveld, E., Monai, M., Ferri, D., Nachtegaal, M., Unocic, R., van Santen, R. A., Frenkel, A., Meirer, F., Weckhuysen, B. M.; Manuscript in Preparation.
Powered by Eventact EMS