
Synthesis of octastannacubane from tin hydride via distannene
Recently, we found that intermediate bis-silyl stannylene (2) is convenietly generated under mild conditions (70°C) from tin hydride (1) (Scheme, step a). Stannylene 2 dimerizes instantaneously yielding the distannene (3) (Scheme, step b). When 2 is generated in the presence of NHCMe, it is quantitatively converted to the stable stannylene-carbene complex (4) (Scheme, step c).
Heating of distannene 3 at 70°C for 24 hours yields the known stable stannyl radical (5) and surprisingly also octastannacubane (6) (Scheme, step d). The formation of radical 5 (which can be isolated) along with by – products attributed to silyl radical (7) (Scheme, step e), suggest that 6 is obtained via a radical mechanism via homolytic cleavage of the Si-Sn bond in 3. Radical 7, generated in the thermolysis of 3, can be trapped by radical scavengers, such as C60. (Scheme, step f).
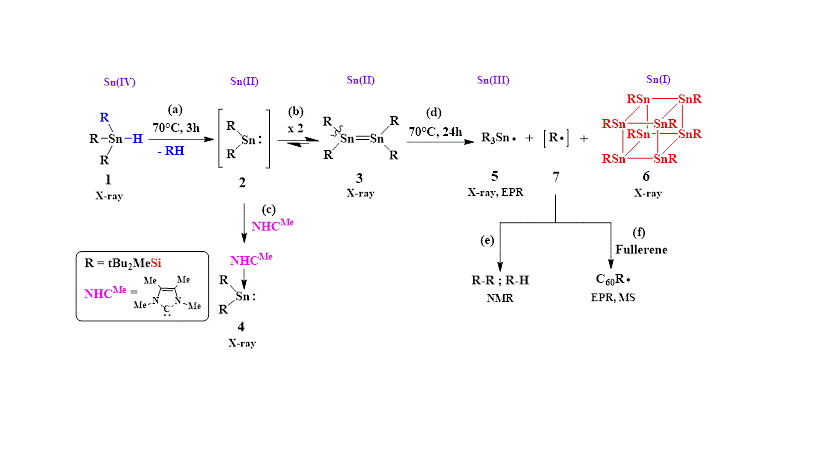
Scheme
Powered by Eventact EMS