
Developmental and Behavioral Follow-up at Age 2 Years of Preterm Children Supplemented with Docosahexaenoic and Arachidonic Acid at Age 1 Year: The Omega Tots Trial
2Center for Biobehavioral Health, Nationwide Children's Hospital, USA
3Epidemiology, The Ohio State University College of Public Health, USA
4Crane Center for Early Childhood Research and Policy, The Ohio State University, USA
5Pediatrics, Pennsylvania State University College of Medicine, USA
6Center for Perinatal Research, Nationwide Children's Hospital, USA
7Obstetrics and Gynecology, The Ohio State University College of Medicine, USA
8Alberta Children's Research Institute, University of Calgary, Canada
9Hotchkiss Brain Institute, University of Calgary, Canada
10Internal Medicine, The Ohio State University College of Medicine, USA
Background: Toddlers consume low amounts of docosahexaenoic acid (DHA) post-weaning. The Omega Tots randomized, blind, placebo-controlled trial of children born at
Objective: To determine whether daily DHA and arachidonic acid (AA) supplementation at age 1 year improves development and behavior at age 2 years among children born preterm.
Methods: Omega Tots participants were followed up at 26-35 months’ chronological age to assess longer-term outcomes. Parents remained blind to treatment assignment and completed the Developmental Profile-3 (global cognitive); MacArthur-Bates Communicative Development Inventory (expressive language); Behavior Rating Inventory of Executive Function (BRIEF-P); Child Behavior Checklist 1.5-5 ADHD, PDD, attention; and reported diagnoses. Analyses were intent-to-treat. Mixed models compared treatment groups, controlling for clustering and baseline scores whenever possible. Sub-group effects for language by birthweight and for executive function by income were explored because adverse effects were observed in the original trial.
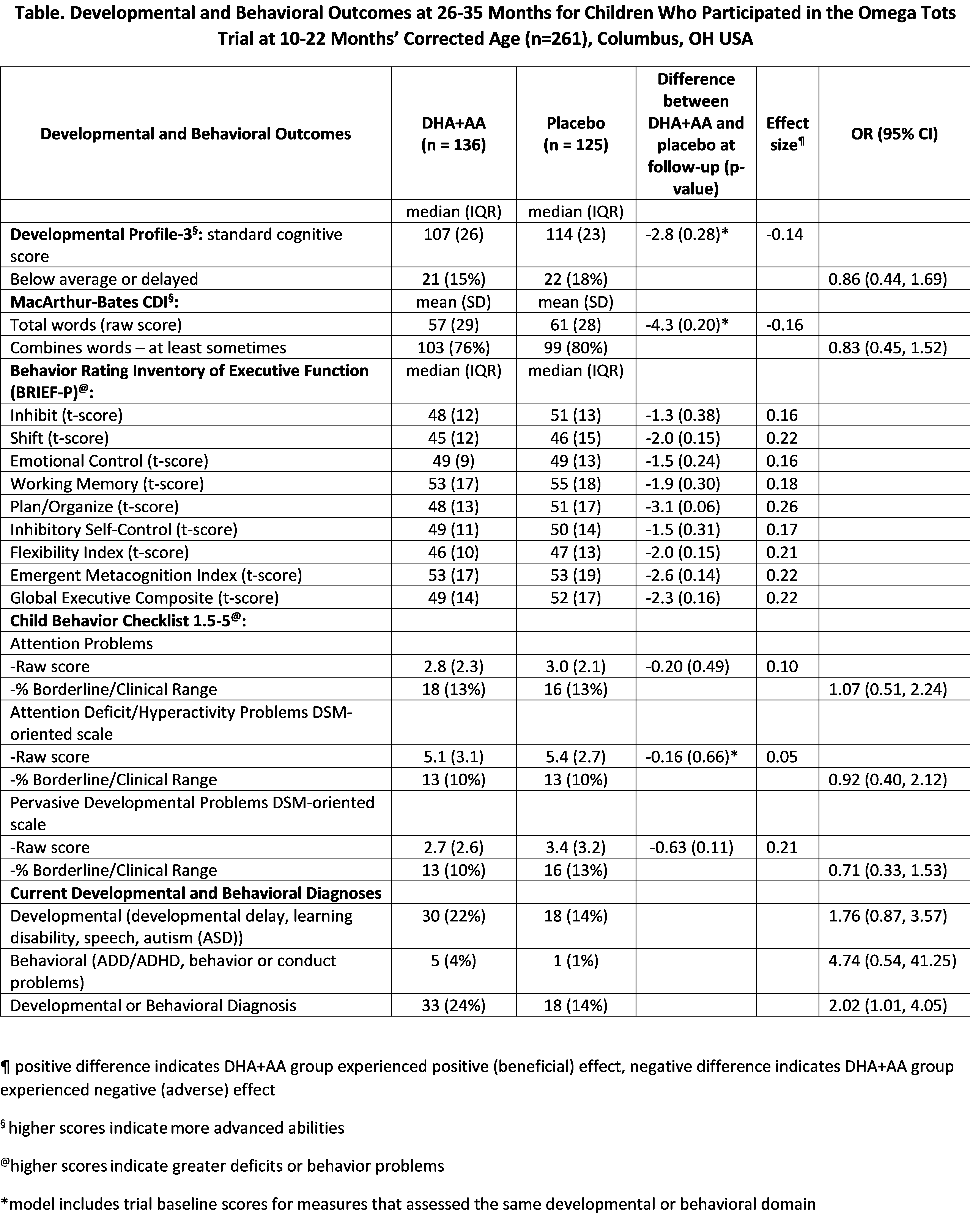
Results: Parents of 261/328 (80%) eligible children participated. No benefits of supplementation were observed at age 2 (see Table). The DHA+AA group had lower standard cognitive scores than the placebo group, but this was not statistically significant (median=107 vs 114, P=0.28). The DHA+AA group was twice as likely to have a current developmental/behavioral diagnosis (OR=2.02, 95% CI: 1.01, 4.05). In exploratory sub-group analyses, children with birthweight P=0.03, effect size=-0.44). Effects observed in the original trial on executive function by income were no longer present.
Conclusion: Long-term, robust assessment is needed to clarify the long-term effects of DHA+AA supplementation in toddlerhood for children born preterm.
Powered by Eventact EMS