
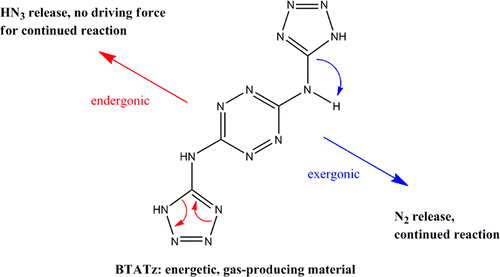
Proposed proton-transfer mechanism for the initial decomposition steps of BTATz
2RAFAEL, Advanced Defense Systems Ltd., Haifa, Israel
The first steps in the gas-phase decomposition mechanism of N3,N6-bis-(1H-tetrazol-5-yl)-1,2,4,5-tetrazine-3,6-diamine, BTATz, anions and the kinetic isotope effects in these processes were studied using combined multistage mass spectrometry (MS/MS) and computational techniques. Two major fragmentation processes, the exergonic loss of nitrogen molecules and the endergonic loss of hydrazoic acid, were identified. The observation of a primary isotope effect supported by calculations suggests that the loss of a nitrogen molecule from the tetrazole ring involves proton migration, either to or within the tetrazole ring, as a rate-determining step. The fragmentation of a hydrazoic acid occurs through an asymmetrical retro-pericyclic reaction. Calculations show the relevance of these mechanisms to neutral BTATz. Our findings may contribute to the understanding of decomposition routes in these nitrogen-rich energetic materials and allow tailoring their reactivity and decomposition pathways for better control of performance.
Powered by Eventact EMS