
Catalytic enantioselective synthesis of CF3-substituted ethers and thioethers. Organo-titanium nucleophiles in asymmetric cross-coupling reactions
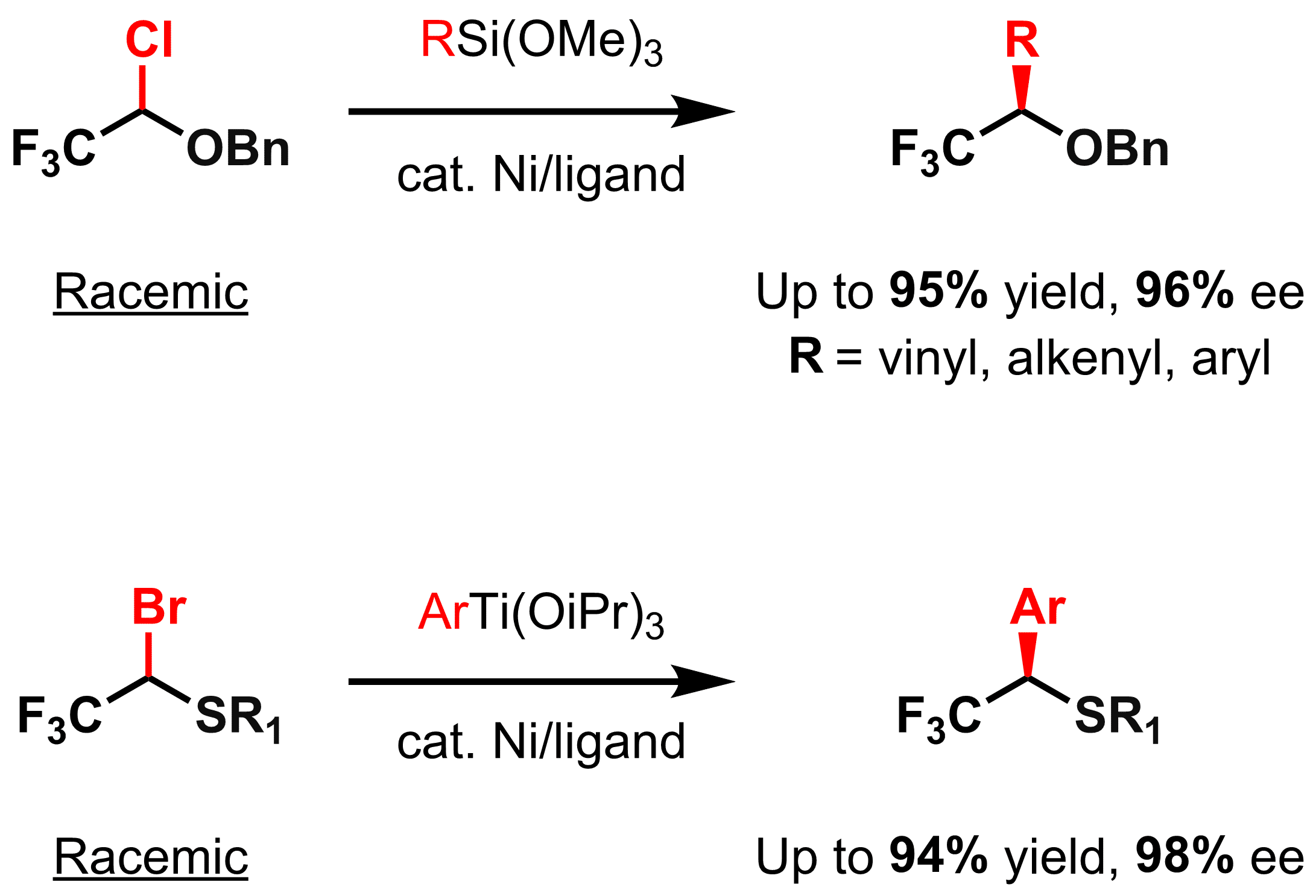
Fig. The reaction scheme
Synthetic approaches towards chiral organic compounds bearing trifluoromethyl-substituted stereocenters are of great interest for agrochemical and pharmaceutical labs and industries in their search for new bioactive materials. We report on employment of bisfunctionalized electrophiles, bearing both a trifluoromethyl and a functional group as direct substituents of the reactive center, in cross-coupling reactions.1 We exemplify this concept in the asymmetric synthesis of enantioenriched α-trifluoromethyl- and perfluoroalkyl-containing (tio)ethers and alcohols by nickel-catalyzed stereoconvergent cross-coupling reaction. Substrate electrophiles are conveniently prepared in few steps from trifluoroacetic acid. The method represents a conceptually new approach to chiral CF3-substituted alcohols and (thio)ethers and allows for a rapid catalytic preparation of a wide range of these valuable compounds in high yields and enantioselectivity.
An ability to employ a broad spectrum of transmetallating partners (organoboron, -silicon, -tin, -zinc, -magnesium, etc.) in cross-coupling reactions, made these transformations highly versatile and adaptive to various functional groups and reaction conditions. While scarce examples of organotitanium nucleophiles in cross-couplings are known, their use in asymmetric variants, to the best of our knowledge, is not reported. Here we describe the first example of utilization of organotitanium transmetallating agents in asymmetric cross-coupling reaction employed for the synthesis of enantioenriched α-CF3 thioethers.
- Varenikov A., Gandelman M.; Nature Communications 9, 3566 (2018).
Powered by Eventact EMS