
Influence of anionic ligand exchange in latent sulfur-chelated ruthenium precatalysts
While the impact of the NHC and alkylidene ligands on olefin metathesis catalysis has been extensively studied for their ruthenium complexes, the influence of the anionic ligands is less recognized. A recent study from our group has revealed four new cis-dianionic S-chelated ruthenium benzylidenes.1 The cis-dichloro geometry imposed by the S-chelation facilitates ligand exchange; thus, Ru-S-Cl was mixed with different carboxylate silver salts to exchange both chloride ligands. Ru-S-TFA, Ru-S-Gly and Ru-S-Ala were obtained in good yields and remained in the cis-dianionic configuration. Moreover, in order to obtain the iodide analogue, Ru-S-Cl could be simply mixed with NaI.
The new complexes were tested for their RCM activity. Ru-S-Cl and Ru-S-I produced a full conversion to the RCM product in hot toluene. Unexpectedly, the carboxylate complexes produced only the cycloisomerization product and in low conversions. In addition, changing the solvent to THF (65°C) completely reversed the selectivity of the Ru-S-Cl precatalyst. Thus, we discovered that just by changing the solvent the catalytic selectivity under thermal activation can be completely changed, from metathesis in toluene to cycloisomerization in THF.
Curiously, while the ROMP reaction of 1,5-cyclooctene (COE) using 1 mol% Ru-S-Cl provided 100% conversion in 1h, Ru-S-I was completely inert in this case. The carboxylate complexes, on the other hand, promoted migration of the monomer’s double bond to produce 1,4-cyclooctadiene. This interesting behavior is currently under further study.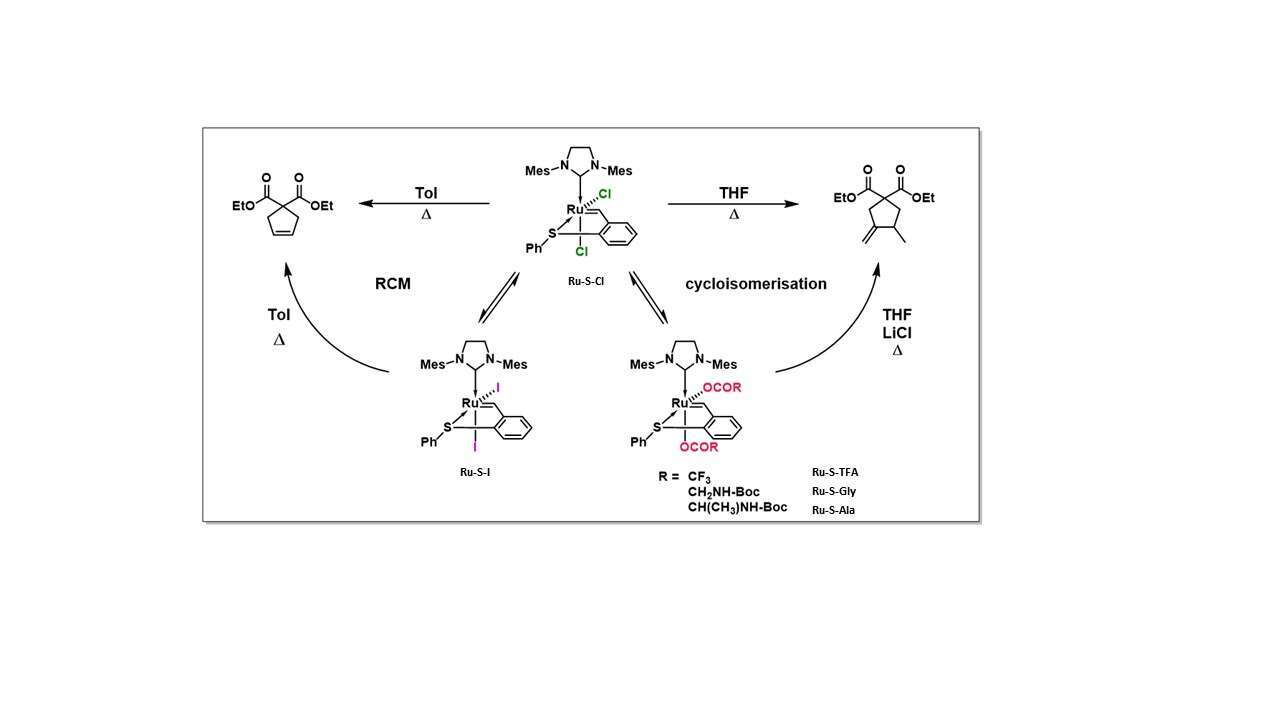
1 E. Ivry, N. B. Nechmad, M. Baranov, I.l Goldberg, and N. G. Lemcoff, Inorg. Chem. 2018, ASAP, DOI: 10.1021/acs.inorgchem.8b0291
Powered by Eventact EMS