
Carbide-carbon electrocatalysts for hydrazine oxidation: The surprising effect of doping on nanostructure
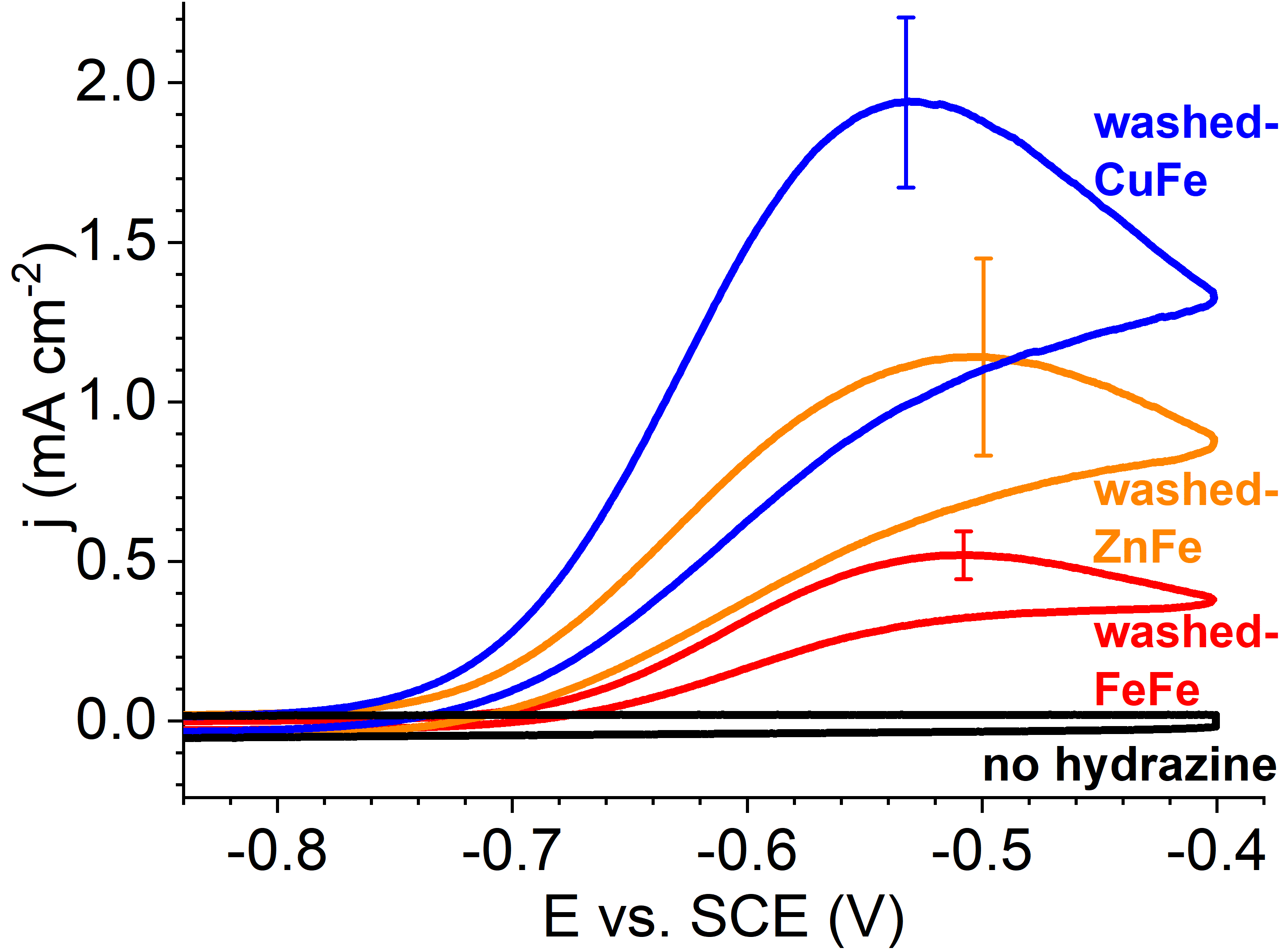
We have recently reported a new composite electrocatalyst for the hydrazine oxidation reaction (HzOR). It is composed of doped carbide nanoparticles embedded in a N-doped C matrix. Following this discovery, we became interested in understanding the effect of doping on the HzOR activity of the composite. To this end, we investigated a homologous series of pyrolysis precursors, based on the same basic structure, yet with different doping counterions. We prepared three bimetallic pentanuclear cluster complexes, namely [(Fe(2,2’-bipy)2)3(Fe(CN)6)2], [(Zn(2,2’-bipy)2)3(Fe(CN)6)2], and [(Cu(2,2’-bipy)2)3(Fe(CN)6)2]. Upon pyrolysis, they formed a carbon matrix imbedded with metallic phases, Fe3C and traces of Fe2O3 (abbreviated pyrolyzed-MFe [(M=Fe, Zn, Cu]). Washing removed most of the metallic phases and oxides, leaving mainly Fe3C-imbedded N-doped carbon composite (abbreviated washed-MFe [(M=Fe, Zn, Cu]). We discovered a significant doping effect – focused on nanostructuring the carbon material. Two different dopants – Cu and Zn – induced different pore structures on the micro and meso levels, apparently through different structure-inducing mechanisms. The materials exhibited excellent HzOR electrocatalysis, with some of the lowest overpotentials reported for carbon-based electrocatalysts. Doping was found to have a profound effect on electrocatalysis, through combined mechanism of active site exposure and the formation of flow-enabling mesopores.
Powered by Eventact EMS