
Anchimeric assistance enabled diastereoselective ring opening of fully substituted cyclopropanes via Friedel-Crafts alkylation
Schulich Faculty of Chemistry, Technion – Israel Institute of Technology, Haifa, Israel
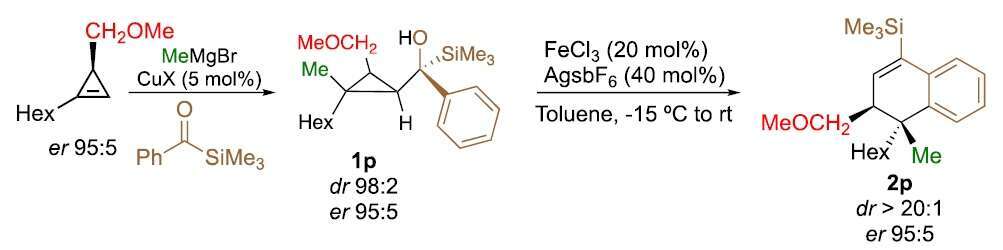
a protocol for the diastereoselective preparation of functionalized dihydronapthalene cores, useful precursors of diterpenoids, was reported from easily accessible cyclopropanes in three catalytic steps from commercially available alkynes. The versatility of the transformation was illustrated by the complete selectivity of the ring-expansion and by the retention of configuration at the stereogenic center during the migration. Mechanistic control experiments reveal the key role of the coordinating group in the overall process.
Powered by Eventact EMS