
Water-soluble CuO nano-core: Synthesis, characterization and catalysis
Abstract: An unprecedented role for metal-oxide cluster-anions (polyoxometalates, or POMs) as covalently coordinated inorganic ligands for individual anatase nanocrystals and hematite nanocrystals, gives isolable anionic clusters uniquely positioned between molecular macroanions and traditional colloidal nanoparticles.[1],[2] POM anion K8Nb6O19 serves as tridentate “capping” ligand for complexed Cu(II) ions linked to CuO (tenorite) cores. Different spectroscopic methods, electron microscopic images, and analytical measurements confirm the presence of POM-capping ligand, covalently bound to the surfaces of the 3-6 nm tenorite cores. On illumination with a visible light, the hybrid NCs oxidized water in presence of S2O82- as a sacrificial oxidant (Figure 1). Indefinite stability of these unique complexes in water over a wide range of pH values, including the isoelectric point of tenorite, led us to study the photochemical water oxidation in different pH values. Kinetic study; dependence of photochemical O2 production rate with respect to the concentration of [NCs] and [S2O82-] is in progress to understand the mechanism of water oxidation.
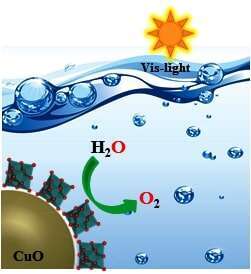
Figure 1. Visible-light-driven water oxidation by the water-soluble complex of tenorite.
References:
- M. Raula, G. Gan Or, M. Saganovich, O. Zeiri, Y. Wang, M. R. Chierotti, R. Gobetto, I. A. Weinstock, Angew. Chem. Int. Ed. 2015, 54, 12416-12421.
- Biswarup Chakraborty, Gal Gan-Or, Manoj Raula, Eyal Gadot & I. A. Weinstock, Nature Communications, 9, 2018, 4896
Powered by Eventact EMS