
Iridium-catalyzed olefin isomerization as a surprisingly simple solution for the stereoselective synthesis of fully substituted enolates
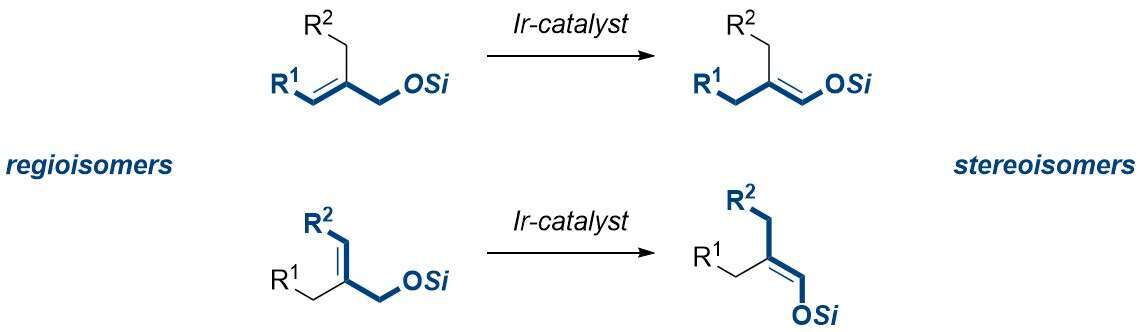
Enolates are probably the most useful synthetic intermediates in organic chemistry, serving as precursors for α-substituted carbonyl compounds, which are widespread and relevant for a range of applications. Traditionally, enolates are formed by deprotonation of the corresponding carbonyl compound bearing a hydrogen atom at the α-position using a strong base at low temperatures. When forming an enolate, the stereoselectivity of the process must be considered (which stereoisomer of the enolate double bond is formed), along with the compatibility of the functional groups present in the substrate with the conditions used. The many methods developed over the last decades reliably deliver enolates with high yield and stereoselectivities when applied to substrates which bear only one substituent at the α-carbon and do not feature base-sensitive functionality. However, the situation changes dramatically when one wishes to stereoselectively form an enolate bearing two, sterically similar substituents at the α-position, as traditional methods fail to provide a solution. Herein, we demonstrate a conceptually different approach towards enolates (specifically, silyl enol ethers), starting from silyl-protected allylic alcohols and using an Ir-based olefin isomerization catalyst, which allows the stereoselective formation of previously elusive, highly substituted enolates. The developed procedure employs low catalyst loadings, tolerates a wide range of common functional groups and affords the products with consistently high yields and stereoselectivities, without requiring column chromatography. The isomerization is believed to proceed through a 1,3-hydride shift mediated by the Ir-catalyst. By exploiting the mechanism and source of stereocontrol in the process, we have been able to selectively form either of the two possible stereoisomers of a given enolate with high streoselectivities, starting from regioisomeric substrates.
Powered by Eventact EMS