
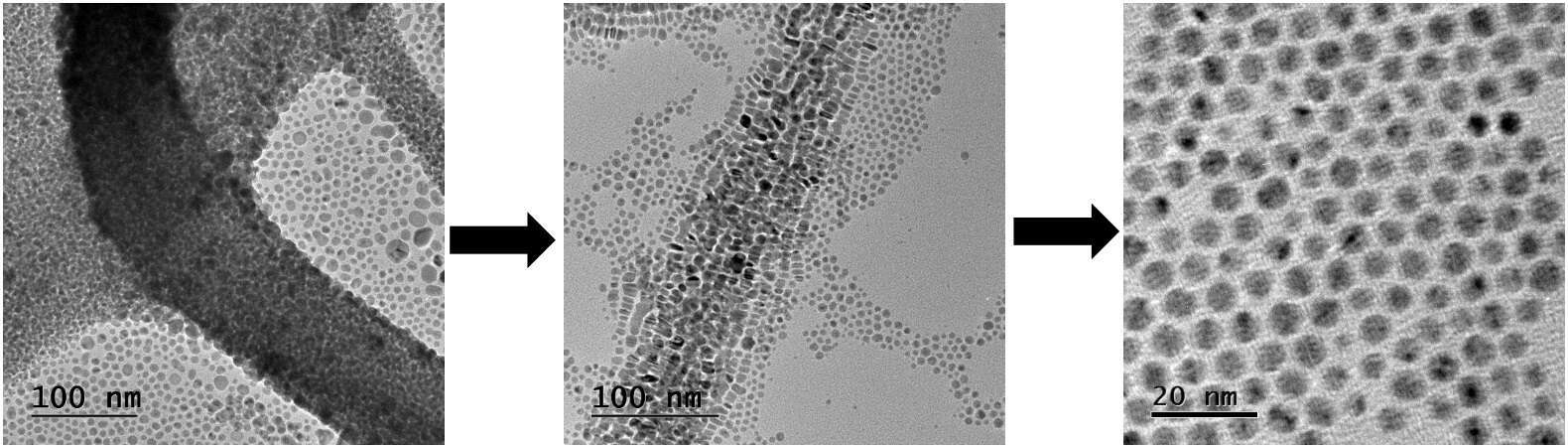
Intermediates in one-step synthesis of silver nanoparticles
Solution synthesis of noble-metal nanoparticles is generally thought to occur via formation of seed clusters by the stronger reducing agent such as borohydride, followed by growth due to the milder reducing agent such as hydrazine.[1] We followed the formation of non-polar 5 nm silver nanoparticles[2] by electron microscopy and UV/Vis absorption spectroscopy. Our study revealed additional intermediates - most notably, flexible fibers with embedded nanoparticles, and aggregates of short silver nanorods - explaining the transient colors of the solution. Understanding mechanisms of nanoparticle formation leads towards rational design of nanomaterials synthesis methods, such as by finding conditions in which an intermediate is metastable.
References:
[1] N. R. Jana, X. Peng, Single-Phase and Gram-Scale Routes toward Nearly Monodisperse Au and Other Noble Metal Nanocrystals, J. Am. Chem. Soc. 2003, 125, 14280.
[2] R. Klajn et al. Bulk synthesis and surface patterning of nanoporous metals and alloys from supraspherical nanoparticle aggregates, Adv. Funct. Mater. 2008, 18, 2763.
Powered by Eventact EMS