
Trifluoromethylation affecting cobalt corroles for electrocatalysis
There is a worldwide effort for replacing the very effective, but absolutely non-sustainable, Pt- and Ir- based catalysts for green energy applications such as oxygen reduction. The biggest success so far is by cobalt complexes, including those chelated by corroles, but there is still an urging need for learning how to tune redox potentials and other fundamental properties thereof. Having exhausted many other options, by us and many others in the field, we hypothesized that CF3-substitution of the macrocycle would induce major beneficial changes.
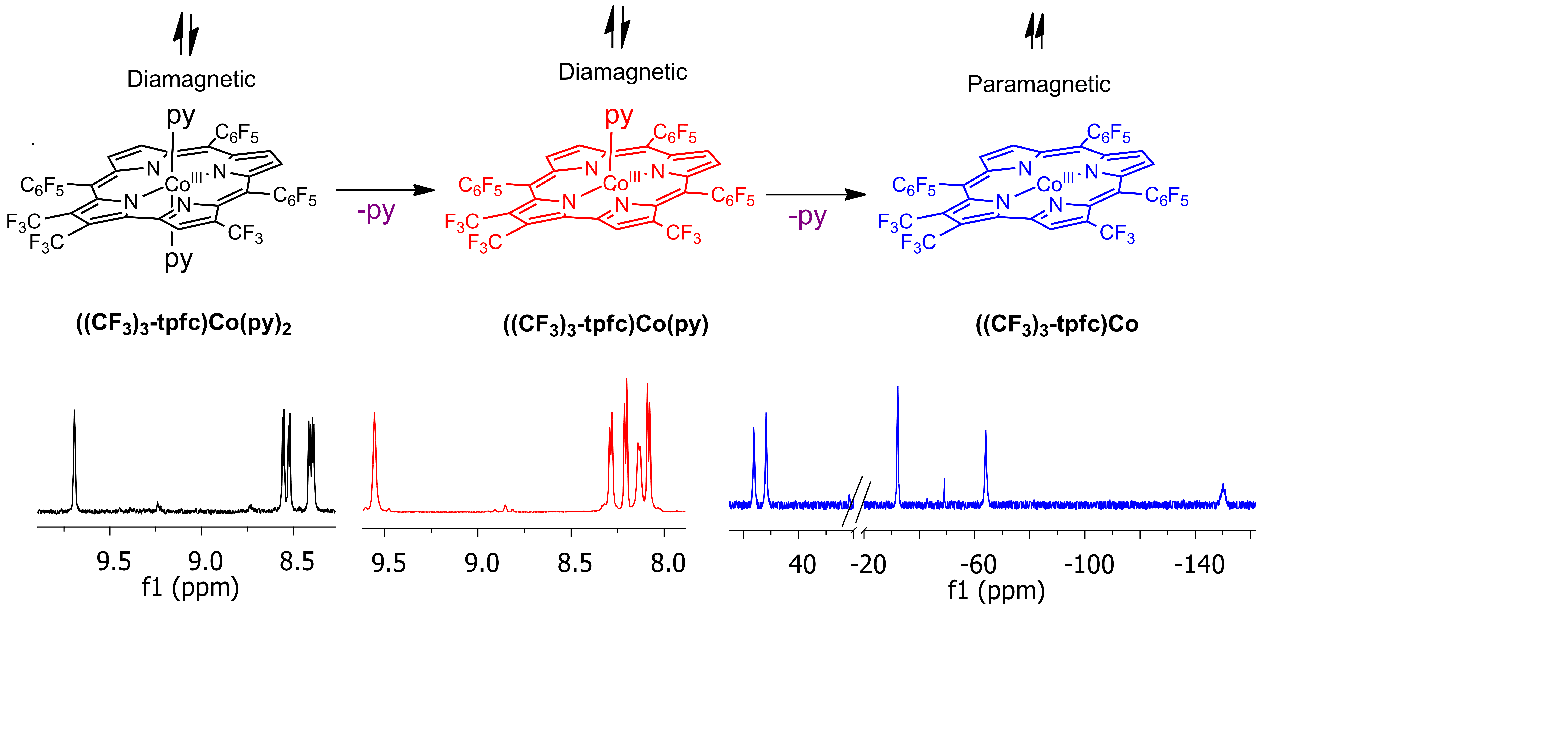
We now describe the resolution of the challenges concerning both the synthesis and the proper characterization of CF3-substitution on particular C atoms, by introducing a one-pot metallation/iodination1 followed by CF3-/I- nucleophilic substitution reactions. This is followed by proving our lead hypothesis correct, i.e., demonstrating the very large effects of the CF3 groups on the structural, electronic and redox properties of the cobalt corroles. The blocking of the most reactive C atoms further allowed for isolation of 6-, 5-, and the most rare 4-coordinate cobalt complexes; and for the demonstration that while the two former are diamagnetic low-spin d6, the latter are paramagnetic due to their intermediate-spin state. Our deduction of how the CF3 groups and the axial ligands affect both the redox potentials and their reversibility is essential for the design of optimal catalysts for two of the most important catalytic processes for clean energy: the conversion of protons to molecular hydrogen and the reduction of oxygen to water. We hence trust that our work will be of outstanding interest and exceptional value much out of the particular complexes that we work on, as the principles are applicable to many other metal-binding ligands.
Powered by Eventact EMS