
Towards silanone via bromosilanols
Stable silanones, R2Si=O, the silicon analogue of ketones, have not yet been isolated.
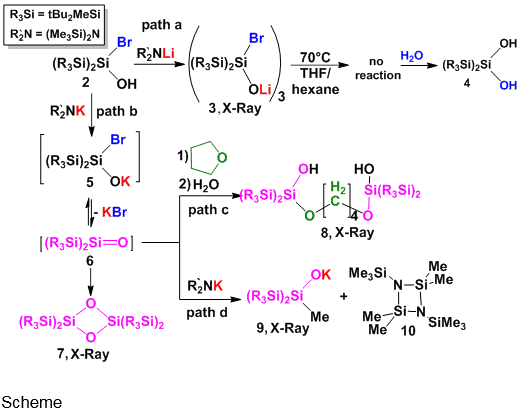
Here, we report the synthesis and X-Ray structural analysis of lithium bromosiloxide 3 and the generation of potassium bromosiloxide 5 which we believe yields a transient silanone showing unique reactivities (path c and d), which were studied by DFT calculations.
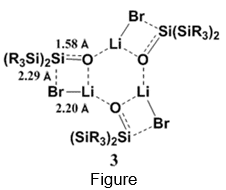
Reaction of 2 with )Me3Si(2NLi yields trimer 3 (path a). X-Ray structural analysis of 3 reveals significant Li-Br interactions, i.e. r(Li--Br) = 2.20 Å (shorter than the sum of their ionic radii), a very short r(Si-O) = 1.58 Å and a relatively long r(Si-Br) = 2.29 Å.(see Figure) These structural features point to a major contribution of a R2Si=O···LiBr complex character in 3. Disappointingly, heating 3 to 70°C in hexane or THF does not lead to LiBr elimination, indicating strong intermolecular bonding in 3.1 Hydrolysis of 3 yields diol 4, the expected product from the reaction of R2Si=O with water.
To force elimination of MBr leading to generation of silanone, lithium was changed by potassium. Bromosilanol 2 reacts with )Me3Si(2NK to yield unstable potassium bromosiloxide 5 which eliminates KBr to yield transient silanone 6 (path b). 6 dimerizes into cyclosiloxane 7, recognized as a dimerization product of silanones. Addition of THF to this reaction and subsequent hydrolysis yields product 8 (path c), which is interpreted as a ring-opening product of THF by the highly reactive transient silanone 6. Reaction of 2 in the presence of two equivalents of )Me3Si(2NK yields methylated product 9 and cycle 10 (path d). 10 is probably a dimer of silaimine - product of demethylation. Proposed mechanisms of both reactions investigated by Density Functional Theory (DFT) calculations will be presented.
Powered by Eventact EMS