
Sequence control of self-assembled peptides for hydrogels design and proton conductance
Molecular self-assembly is an influential appliance for fabrication of functional nanostructures. Particularly self-assembly of amphiphilic peptides in water is the focus of the research due to their ability to form a wide range of functional supramolecular structures1,2. Such molecules composed for example of repetitive dyads of charged and aromatic residues are important for developing new hydrogel materials for various applications in e.g. biomedicine, materials, biotechnology and solar energy. Here we designed and synthesized a set of amphiphilic peptides3,4 each equipped with two different aromatic amino acids residues at positions R1 and R2 (see Figure 1). Probed molecules form hydrogels of different stiffness that relates to their amino acids composition as well as the morphology of the assembled fibers. Additionally described structure/activity relationship (SAR) is further displayed in series of experiments investigating proton conductivity. We found a connection between the amount of intermolecular interactions and the proton mobility: lower proton transport is determined by the higher interference to mobility, resulting from enhanced interactions within the peptides. We suggest that the presented study is significant because these or similar compounds may be used in biomedical applications as injectable materials.
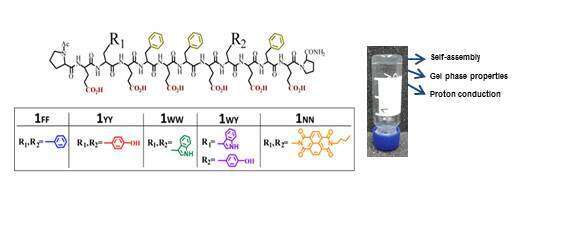
Figure 1. Molecular structure of five peptides that are examined in the described study.
References:
[1] Rubinov B., Wagner N., Matmor M., Regev O., Ashkenasy N. and Ashkenasy G., ACS Nano 6, 9, 7893-7901 (2012).
[2] Nanda J., Rubinov B., Ivnitski D., Mukherjee R., Shtelman E., Motro Y., Miller Y, Wagner N., Cohen-Luria R., Ashkenasy G., Nature Commun 8, 434 (2017).
[3] Ivnitski D., Amit M., Silberbush O., Atsmon-Raz Y., Nanda J., Cohen-Luria R., Miller Y., Ashkenasy G., Ashkenasy N., Angew. Chem. Int. Ed. 55, 9988 (2016).
[4] Ivnitski D., Amit M., Rubinov B., Cohen-Luria R., Ashkenasy N., Ashkenasy G. Chem. Commun. 50, 6733-6736 (2014).
Powered by Eventact EMS