
On the redox chemistry of U(IV) DOTA
2Department of Chemistry, Nuclear Research Center Negev, Beer-Sheva, Israel
3Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
4Departmenf of Chemical Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel
5Radiochemistry and Processes Department, Nuclear Energy Division – CEA, Marcoule, France
![[UIV6(OH)4(O)4(HDOTA)4(H2O)8] cluster](https://events.eventact.com/duin/31312/U6DOTA4.jpg)
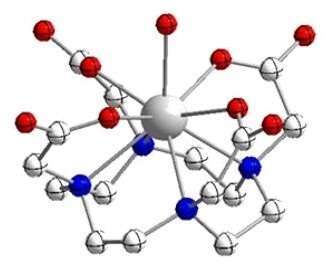
[UIV6(OH)4(O)4(HDOTA)4(H2O)8] UIVDOTA monomer
The 1,4,7,10-tetrazacyclodecane-1,4,7,10-tetraacetic acid macrocyclic ligand (DOTA) is known as a powerful chelating agent for several tervalent Lanthanide1 and Actinide2 ions. Previous attempts to study its chemical reaction with the aqueous air sensitive U(IV) ion, yielded only the oligomeric [UIV6(OH)4(O)4(HDOTA)4(H2O)8] cluster3 which has a distinct UV-VIS absorbance spectrum and a limited solubility at the pH range 0.5-5.3.
Herein we report the U(IV)DOTA monomer synthesis and its aqueous redox chemistry.
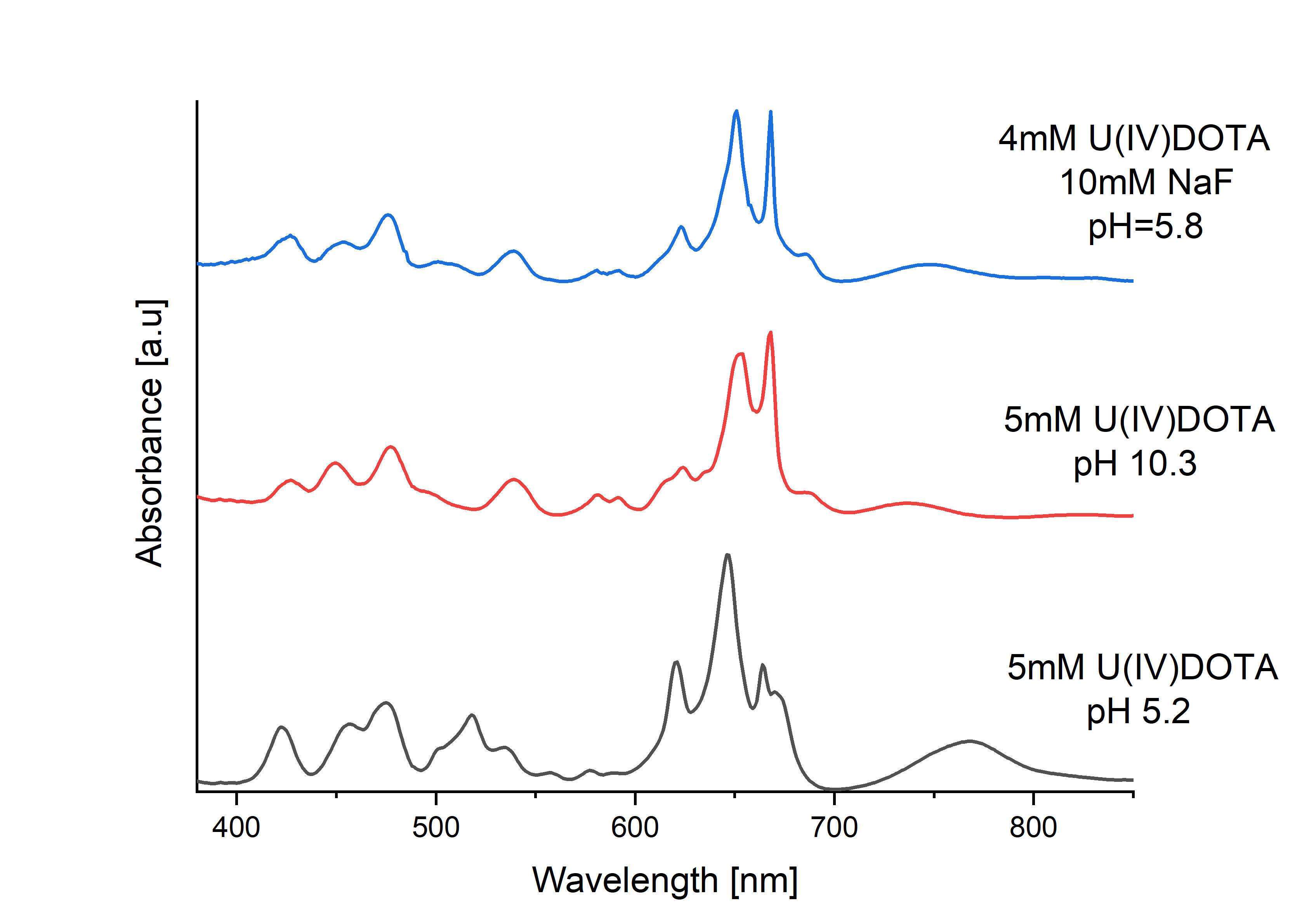
Its reversible chemical modification at neutral and slightly basic pHs and in the presence of fluoride was monitored by 1H-NMR, UV-VIS absorbance spectroscopy and by several electrochemical methods. The complex is exceptionally inert to oxidation by molecular oxygen, does not hydrolyze at neutral and slightly basic pHs and does not undergoes oligomerization as was shown recently for different U(IV)/[UIV6O4(OH)4]12+ systems4, 5. The electrochemical results suggest that an axially coordinated fluoride ligand stabilizes kinetically the pentavalent U(V)DOTA and on the contrary, a coordinated hydroxide ligand facilitates the UIV/IIIDOTA redox process.
References
- Viola-Villegas et al. Coordination Chemistry Reviews, 2009. 253(13): p. 1906-1925.
- Audras et al. Inorganic Chemistry, 2017. 56(20): p. 12248-12259.
- Tamain et al. Chemistry, 2017. 23(28): p. 6864-6875.
- Falaise et al. Inorganic Chemistry, 2017. 56(11): p. 6591-6598.
- Vanagas et al. Inorganic Chemistry, 2018. 57(12): p. 7259-7269.
Powered by Eventact EMS