
The misadventures of methyl radicals
2Department of Chemistry, Nuclear Research Center Negev, Beer-Sheva, Israel
3Radiation Sources and Applications, Department of Chemical Sciences and the Schlesinger Family Center for Compact Accelerators, Ariel University, Ariel, Israel
Methyl radicals, the simplest alkyl radicals, are key intermediates in a wide variety of reactions in biological, chemical and photochemical systems. Dimethyl Sulfoxide (DMSO) is one of the most common reagents used as a precursor for a controlled production of such radicals. Most studies use chemical (e.g. Fenton’s reagent), photochemical or radiolytic (e.g. β/γ irradiation of water) methods to produce hydroxyl radicals, which then react with DMSO in a 2-step mechanism to produce •CH3 radicals. It is then possible to extract mechanistic and kinetic data by analyzing the gas- phase products` (methane and ethane) yield.
Our study focuses on the reactions which occur in solutions of aqueous DMSO at a low steady state concentration of •CH3, using the radiolytic method.
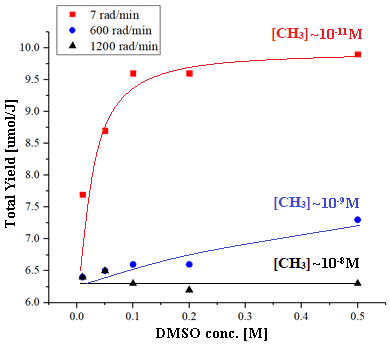
The results show several changes to the known mechanism of the methyl-DMSO reaction. An increased yield of the gaseous products upon reaction with the low steady-state concentration of methyl radicals has been observed (see image), suggesting a chain mechanism. Dimeric products, indicative of a chain reaction, have been detected by high-resolution mass spectrometry. DFT calculations providing thermodynamic and kinetic data were also obtained.
Powered by Eventact EMS