
Mechanistically guided catalyst design for the CuH-catalyzed hydroamination reaction of unactivated olefins
2Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, USA
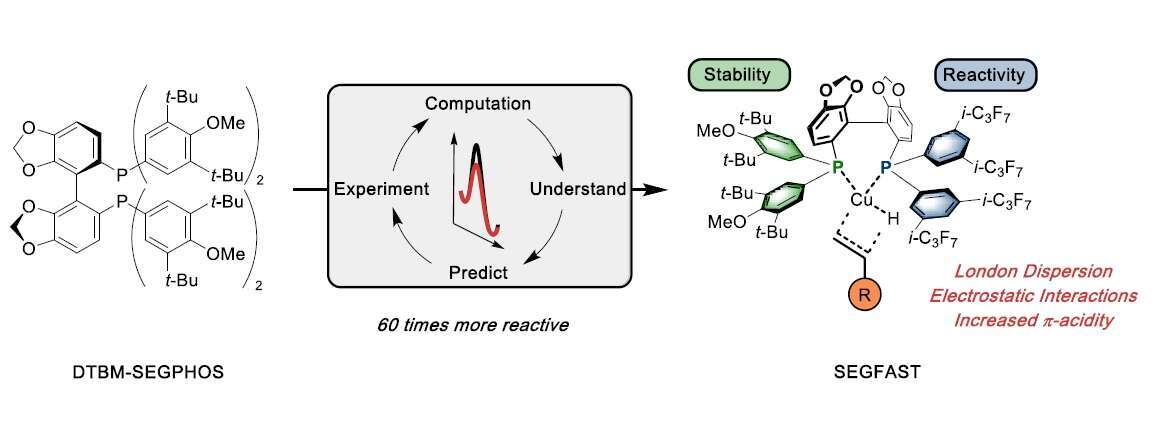
Using a mechanistically guided catalyst design approach, a novel hybrid bisphosphine ligand (SEGFAST) for the CuH-catalyzed hydroamination reaction of unactivated terminal olefins has been developed.[1] Guided by computational prediction and experimental verification the crucial non-covalent interactions necessary for high turnover numbers and catalyst stability were evaluated. It was shown that electrostatic interactions along with London-dispersion forces can significantly lower the activation barrier for hydrocupration leading to a 60-fold rate increase compared to DTBM-SEGPHOS. The unique ability to computationally quantify and experimentally validate the computed ligand scaffolds enabled the optimization of the steric and electronic properties of the ancillary ligand without the need to synthesize and empirically test a large library of ligands.
Moreover, a modular and robust synthetic sequence to access these novel hybrid ligand structures was devised, which allowed for its gram-scale synthesis. Furthermore, the applicability and generality of the new catalyst system was demonstrated, with various olefinic substrates incorporating a variety of functional groups and heterocyclic compounds. Additionally, we believe that this ligand design approach can serve as a platform for future catalyst development
[1] A.A. Thomas, K. Speck, I. Kevlishvili, Z. Lu, P. Liu, S. L. Buchwald, J. Am. Chem. Soc., 2018, 149, 13976–13984.
Powered by Eventact EMS