
Interactions between BIM protein and beta-amyloid may reveal a crucial missing link between Alzheimer`s disease and neuronal cell death
2Bioinorganic Chemistry, Ruhr-Universität Bochum, Bochum, Germany
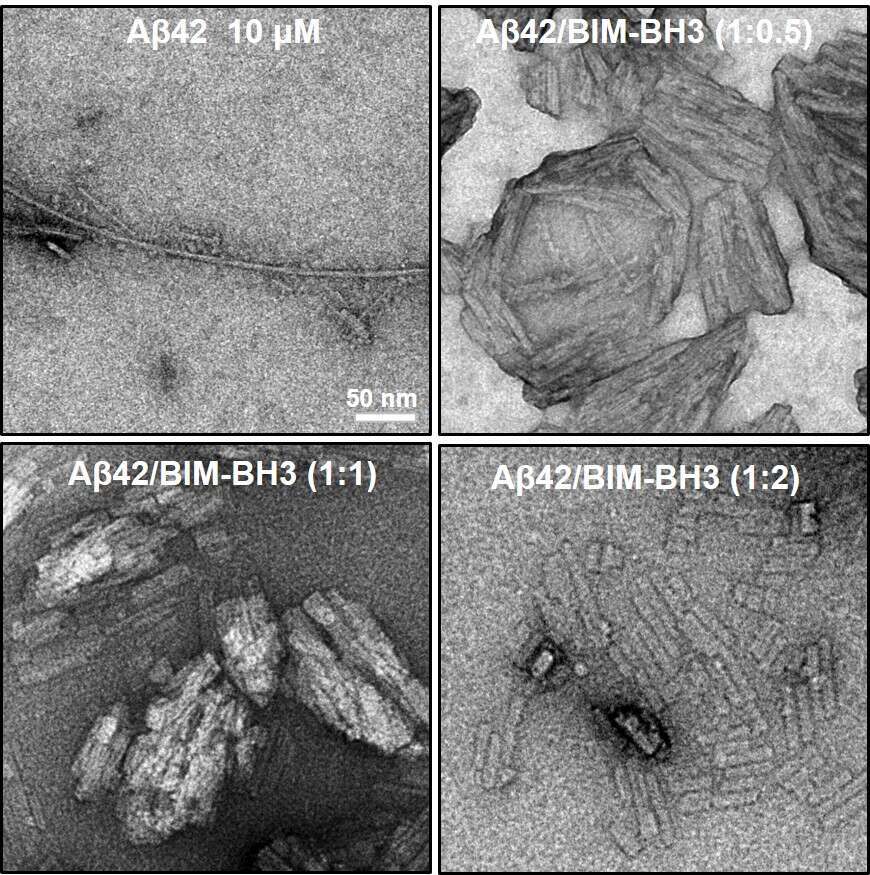
An extensive neuronal cell-death is among the pathological hallmarks of Alzheimer`s disease. While neuron death is coincident with formation of plaques comprising the beta-amyloid (Aβ) peptide, a direct causative link between Aβ (or other Alzheimer`s-associated proteins) and cell toxicity is yet to be found. Here we show that BH3, the primary pro-apoptotic domain of BIM - a key protein in varied apoptotic cascades of which elevated levels have been found in brain cells of patients afflicted with Alzheimer`s disease - interacts with the 42-residue amyloid isoform Aβ42. Remarkably, BH3 modulated the structure, fibrillation pathway, aggregate morphology, and membrane interactions of Aβ42. In particular, BH3 inhibited Aβ42 fibril-formation while simultaneously enhanced proto-fibril assembly. Furthermore, we discovered that BH3-Aβ42 interactions induced cell-death in a human neuroblastoma cell model. Overall, our data is providing a crucial mechanistic link accounting for neuronal cell-death in Alzheimer`s disease patients, and the participation of both BIM and Aβ42 in the neuro-toxicity process.
Powered by Eventact EMS