
A HUMAN DERIVED ANTIMICROBIAL PEPTIDE REVEALED A NOVEL TYPE OF PROTEIN FIBRIL FORMING A SUB-NANO CHANNEL
Antimicrobial peptides (AMPs) are vital key components of the innate immune system. In the last few decades, more than 2000 different AMPs were discovered from all kingdoms of life. Nevertheless, high-resolution atomic structures are available for less than 1% of the AMPs. We determined the crystal structures of human and primate-derived AMPs (named here ‘FunkFibs’) revealing the formation of a novel type of protein fibril composed of four-helical bundles that spiral into a hexameric configuration, forming sub-nano channels along the fibril (Figure A). The single amino-acid difference between the human and primate FunkFibs sequences affected the channel properties which were translated into differences in the levels of antibacterial activity. Visualization of the FunkFibs assemblies using electron micrographs (EM) methods revealed unique and polymorphic shapes including “beads-on-a-string” (figure B) and wide streaky fibers. Moreover, we demonstrated the similarities between the atomic structure to one of the nano-metric entity observed by transmission EM (Figure C and D). Mutagenesis analysis was conducted to analyze the structure-function-fibrillation relationships in these FunkFibs, emphasizing the crucial role of the four-helical bundles in fibrillation (Figure E), nano morphologies and activity. Overall, our work exposed a unique kind of peptide self-assembly that is involved in an antibacterial activity with implications to mechanistic understanding of functional protein fibrils and the design of new antimicrobial drugs.
Figure A
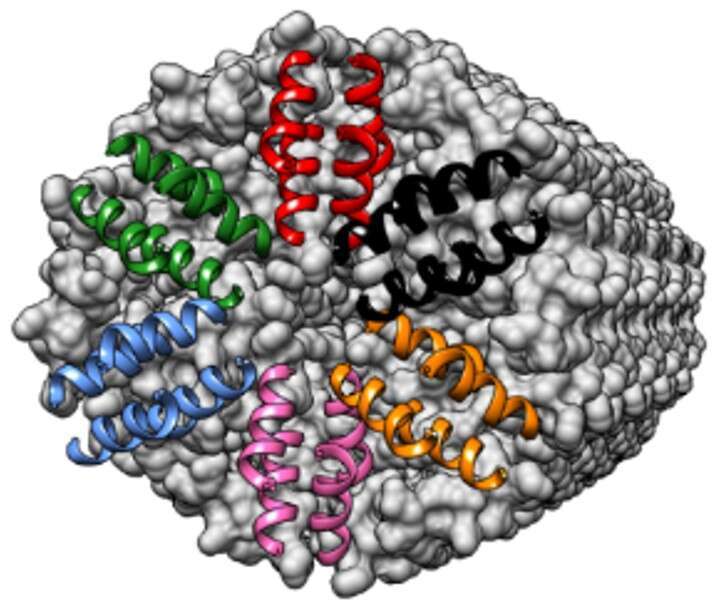
Figure B

Figure C
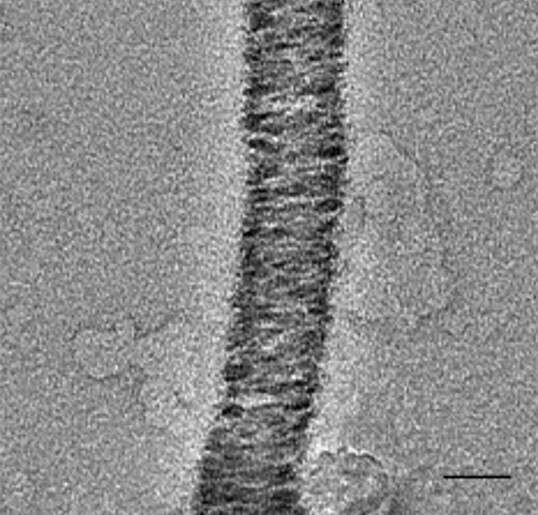
Figure D
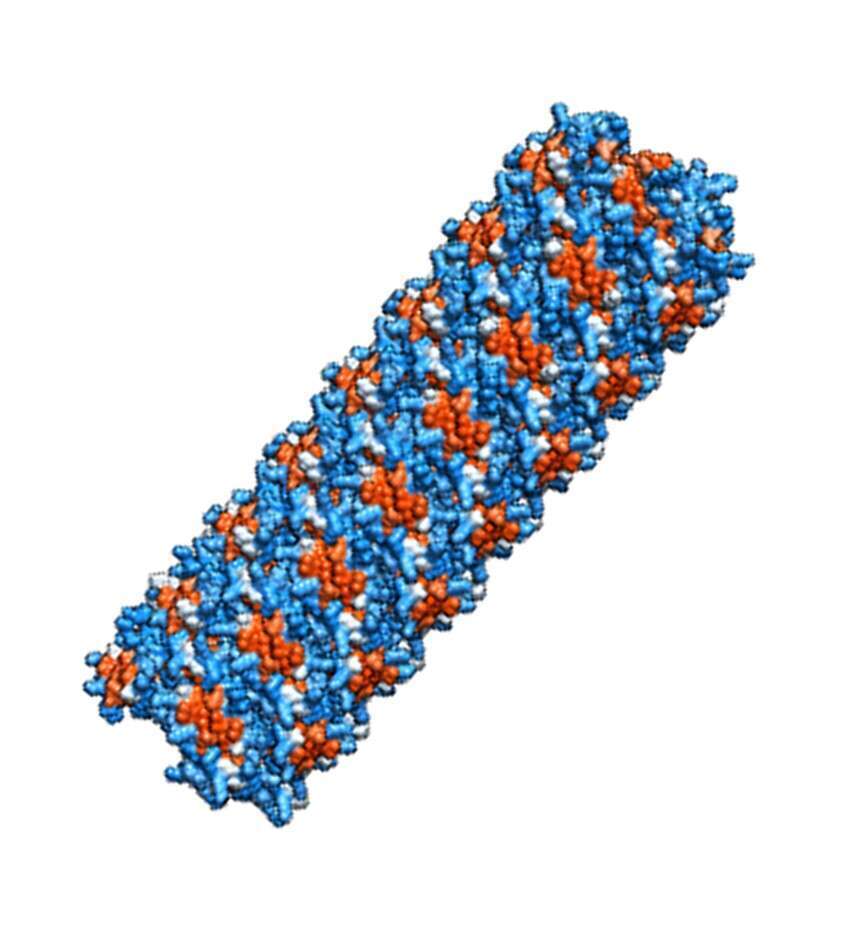
Figure E

Figures A-E: A) Atomic structure of FunkFib; hexameric arrangement of 4-helical bundles (different color), sharing nanochannel in the center and spiral into elongation along a fibril axis. B) Fibrils of "beads on a string", scale bar=200nm. C) A fibril of "worm-like", scale bar=50nm. D) Atomic structure of fibril elongation, colored by hydrophobicity (red), similar to "worm-like" presented in figure C. E) Mutant of FunkFib showing incomplete nanostructures, scale bar=200nm.
Powered by Eventact EMS