
OCTAHEDRAL PtCu NANOCRYSTALS FOR OXYGEN REDUCTION REACTION: ON THE STRUCTURE-PERFOMANCE-DURABILITY RELATIONSHIP BY ELECTRON MICROSCOPY
2Department of Advanced Electron Microscopy, Imaging and Spectroscopy, International Iberian Nanotechnology Laboratory (INL), Braga, Portugal
3Materials Science and Engineering Program, The University of Texas at Austin, Austin, Texas , USA
4Mechanical Engineering Department and IDMEC, Instituto Superior Técnico, University of Lisbon, Lisbon, Portugal
The growing demand for hydrogen-based clean energy has prompted the development of high-performance proton exchange membrane fuel cells (PEMFCs), that convert hydrogen fuel to electricity. The sluggish kinetics on the cathode where the Oxygen Reduction Reaction (ORR) requires improvements of the ORR catalyst cost and durability to become a useful technology. Platinum and Pt-alloys are the most used and available catalysts materials. One of their disadvantages is the high material cost, leading several research groups to develop more efficient materials that will lower the amount of platinum loading on the catalyst.
In this work, we have investigated octahedral Pt-Cu nanocrystals from colloidal synthesis. Several batches of PtCu supported on Vulcan carbon 72XC were produced to study thermodynamic and kinetics based synthesis parameters that influence the shape of the nanoparticles. The parameters can induce the transition from sharp octahedral to concave octahedral shapes. Furthermore, we have analyzed the dependence of the ORR performance on the morphology of the octahedral nanocrystals. In addition, the stability of the material was studied after durability tests. After 4000 cycles in acidic medium under argon saturation or under oxygen, the octahedral morphology of the samples was conserved, showing promises for an application in fuel cells. This work involved the characterization of the materials by rotation disk electrode (RDE) and by high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM on an aberration-corrected FEI TITAN Themis operated at 200 kV), X-ray diffraction (XRD), Transmission Electron Microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS) and X-ray Fluorescence (XRF).
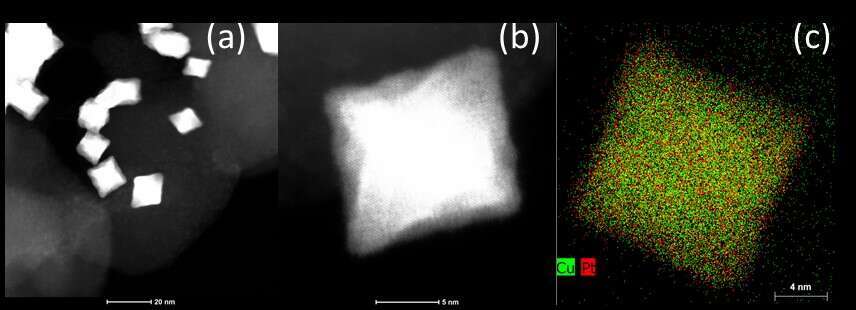
Figure 1. (a) HAADF STEM image of PtCu nanoparticles supported on carbon after 4000 electrochemical cycles, (b) High magnification STEM image of the PtCu nanoparticles. Conditions: 200kV, spot size 9, Conv. angle 17.9mrad. (b) Chemical map of Cu and Pt by EDS
Powered by Eventact EMS