
EXAMINING THE CRYSTAL BOUNDARY OF HEMOZOIN BY DIFFRACTION CONTRAST IN CSTET
2Department of Bimolecular Sciences, Weizmann Institute of Science, Rehovot, Israel
3Faculty of Medicine, The Hebrew University of Jerusalem, Jerusalem, Israel
Malaria is caused by the protozoan parasite - Plasmodium spp., which have a complex life-cycle alternating between the female Anopheles mosquito and the human host. Following a brief asymptomatic phase within the hepatocytes, the parasites invade red blood cells (RBCs) where they undergo asexual multiplication and produce the classical symptoms. Specifically, the parasites digest the hemoglobin within infected red blood cells, but the heme so released is toxic to the parasite itself. Detoxification depends on rapid sequestration of heme to physiologically insoluble crystals of hemozoin. Many anti-malarial drugs interfere with hemozoin crystallization, leading to death of the parasite.
The crystallization environment of hemozoin has been hotly debated. Due to the hydrophobic nature of heme, it was proposed that crystallization should take place within a sub-phase of lipid drops in the parasite digestive vacuole. However, soft X-ray cryo-tomography found no evidence of such a sub-phase or lipid surrounding hemozoin (1). Here we complement those findings using Cryogenic Scanning Transmission Electron Tomography (CSTET). Based on scanning transmission electron microscopy (STEM), contrast reflects the scattering of electrons away from the incident illumination direction as a focused electron probe is rastered across the specimen. Three dimensional reconstructions are generated from tilted projection images.
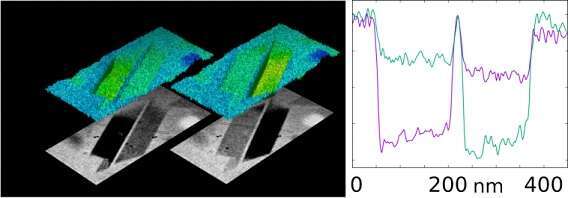
Crystals become dark for particular tilt angles at which conditions for Bragg diffraction are met. This provides a unique opportunity to examine the boundary, similar to viewing the solar corona during an eclipse. CSTET of intact parasites as well as burst cells provide clear evidence of a sharp crystal-aqueous interface without a lipid intermediary.
1. Kapishnikov S, et al. (2012) Oriented nucleation of hemozoin at the digestive vacuole membrane in Plasmodium falciparum. PNAS 109(28):11188–11193.
Powered by Eventact EMS