
A PUTATIVE INHIBITORY DIVALENT CATION BINDING SITE ON TYPE 1 RYANODINE RECEPTOR
2Department of Life sciences, The National Institute for Biotechnology in the Negev, Ben-Gurion University of the Negev, Beer-Sheva, Israel
3The National Institute for Biotechnology in the Negev, Ben-Gurion University of the Negev, Beer-Sheva, Israel
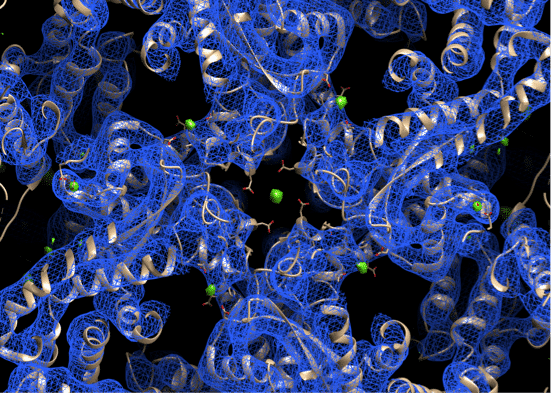
Ryanodine receptors (RyR) are intracellular cation channels that provide a pathway to release Ca2+ ions from intracellular stores into the cytosol, which is essential for numerous cellular functions including excitation-contraction (EC) coupling in striated muscles. They are comprised of four identical ~565 KD protomers, regulatory proteins such as FKBP12/12.6 and calmodulin, enzymes and their respective target proteins in a huge macromolecular complex.1,2 RyRs are activated by calcium ions and ATP3 and are inhibited by high (1mM) calcium concentrations and by magnesium ions (Mg2+).4 1mM Mg2+ is sufficient to reduce RyR channel open probability in lipid bilayer. Lack of sufficient structural information has impeded our understanding of RyRs function and regulation. The closed state structure of type 1 RyR (RyR1) has been solved by single particle cryo-electron microscopy (Cryo-EM).5 And the gating mechanism was revealed by a series of activated RyR1 structures.3 Here we have reconstructed a 3D cryo-EM map of type 1 Ryanodine receptor (RyR1) in presence of magnesium ion (1mM) and we have identified a potential magnesium ion-binding site. We anticipated that Mg2+ ions are locked between two highly conserved negatively charged residues, coming from two different protomers. This new Cryo-EM map suggests a structural basis for divalent cations inhibition of RyR1 channel activity.
REFERENCES:
- Marx, S. O. et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell 101, 365–376 (2000).
- Reiken, S. et al. channel ( ryanodine receptor ) in skeletal muscle : defective regulation in heart failure. 919–928 (2003). doi:10.1083/jcb.200211012
- des Georges, A. et al. Structural Basis for Gating and Activation of RyR1. Cell 167, 145–157.e17 (2016).
- Meissner, G., Darling, E. & Eveleth, J. Kinetics of Rapid Ca2+ Release by Sarcoplasmic Reticulum. Effects of Ca2+, Mg2+, and Adenine Nucleotides. Biochemistry 25, 236–244 (1986).
- Zalk, R. et al. Structure of a mammalian ryanodine receptor. Nature 517, 44–49 (2015).
Powered by Eventact EMS