
Targeting calcineurin-NFAT system: Development of optimized dual-peptide immunosuppressant using proteins directed-evolution
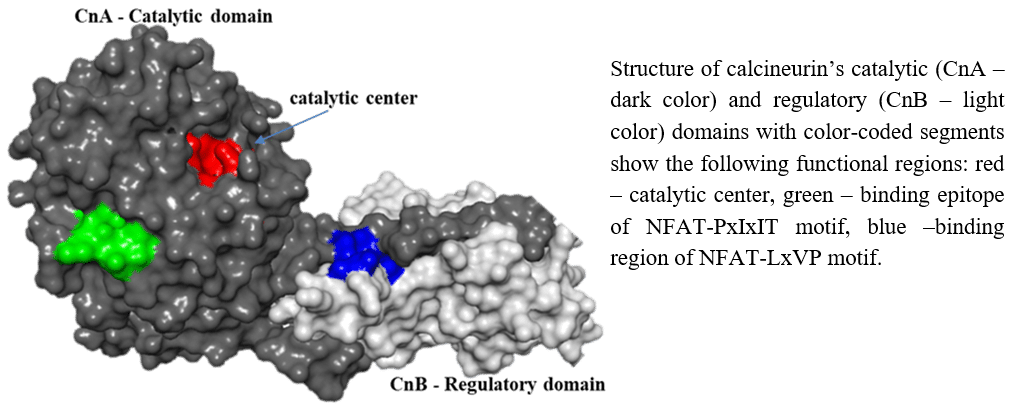
The serine/threonine phosphatase calcineurin targets the nuclear factors of activated T cells (NFATs) and leads to the activation of cytokine genes. Calcium influx activates calcineurin to dephosphorylate multiple serine residues within the 400 residue NFAT homology region; this triggers NFAT nuclear translocation. The binding of the two proteins relies on the interaction between two sites in calcineurin, harboring the conserved motifs PxIxIT and LxVP, located at the N- and C-terminal to the phosphorylation sites in NFAT’s homology region. NFAT`s PxIxIT motif binds calcineurin`s catalytic domain at a location that is a distance from the catalytic site of calcineurin. The LxVP motif binds at the interface of calcineurin`s regulatory and catalytic domains; however, it can also bind to the same site on the catalytic domain as the PxIxIT, but with a lower affinity. The vast range of cellular processes regulated by the calcineurin-NFAT interaction has aroused great interest in the discovery of protein-protein interaction inhibitors that will interfere with the calcineurin-NFAT complex formation while keeping calcineurin`s catalytic site free. Recently, we developed a new way to monitor the heterodimer calcineurin, i.e., catalytic and regulatory domain, with a NFAT homology region that includes the phosphorylation sites flanked by PxIxIT and LxVP binding motifs. This experimental scheme provides valuable data regarding the interaction of the two binding motifs of NFAT with calcineurin. Based on this setup, an optimized dual-peptide constructs by fusion fragments derived from the PxIxIT and LxVP motifs. Furthermore, directed-evolution is being used for the optimization of this chimeric peptide and for the creation of a novel protein-protein interaction inhibitor that can act as a new immunosuppressant.
Powered by Eventact EMS