
Imaging and tracking of exosomes within the brain using gold nanoparticles
2Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel
Exosomes are emerging as effective therapeutic tools for various pathologies. These extracellular vesicles can bypass biological barriers, including the blood-brain barrier, and can serve as powerful drug and gene therapy transporters. However, the progress of therapy development is impeded by several challenges, including insufficient data on exosome trafficking and biodistribution and the difficulty to image deep brain structures in-vivo. Herein, we established a method for noninvasive in-vivo neuroimaging and tracking of exosomes, based on glucose-coated gold nanoparticle (GNP) labeling and computed tomography (CT) imaging. Labeling of exosomes with the GNPs was achieved directly via an active, energy-dependent mechanism that is mediated by the glucose transporter GLUT-1 and involves endocytic proteins. Next, we determined optimal parameters of size and administration route; we demonstrated that 5 nm GNPs enabled improved exosome labeling and that intranasal, compared to intravenous, administration led to superior brain accumulation and thus enhanced in-vivo neuroimaging. Furthermore, we used this technique to track the migration and homing patterns of intranasally administrated Mesenchymal stem cell-derived exosomes (MSC-exo) in various brain pathologies including stroke, autism, Parkinson`s disease and Alzheimer`s disease. Our data demonstrate that MSC-exo have remarkable migration and homing abilities toward specific areas of neuropathology (figure), specifically to neurons, and that inflammation plays a role in these homing mechanisms. Thus, this exosome labeling technique can serve as a powerful diagnostic tool for various brain disorders and could potentially enhance exosome-based treatments for neuronal recovery.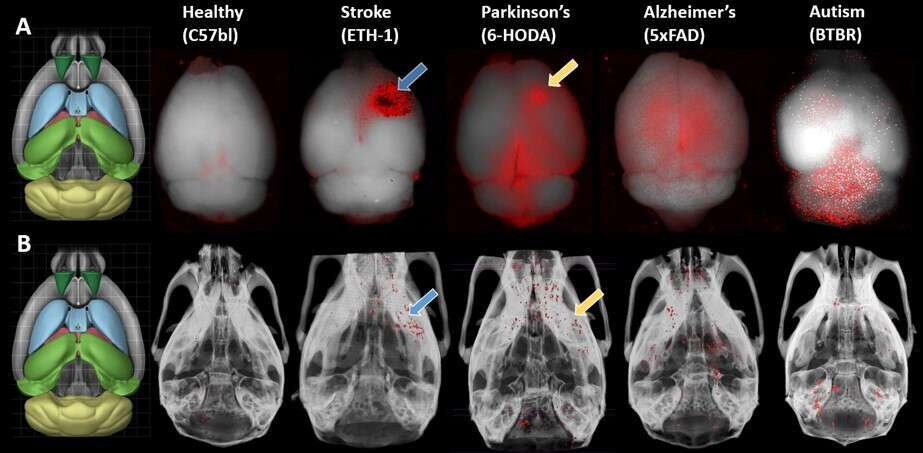
Powered by Eventact EMS