
Invited
New Applications of Proline to Organic Synthesis
Over the past few decades, tremendous progress has been made in the development of protocols for molecular functionalization, revolutionizing the logic of chemical synthesis and opening new ways to prepare complex molecular structures (e.g., natural products, biologically important compounds, medicines and drug candidates). Among various activation/derivatization strategies, the carbon−halogenation, and C-H functionalization can be noted as the most prominent.
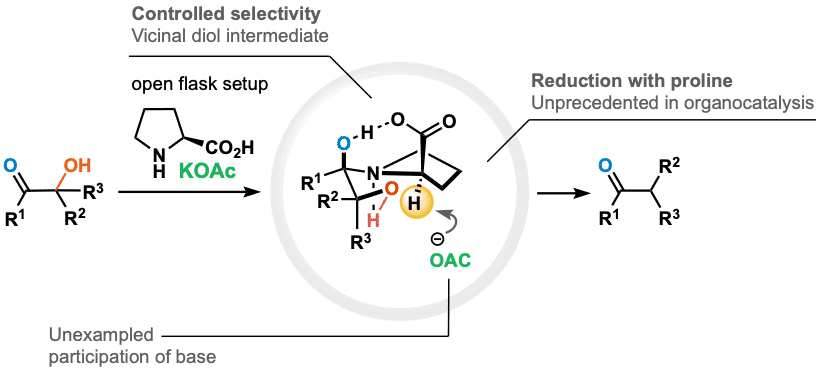
A dehydroxylation through the reduction of α-ketols is another emerging method for a functionalization/derivatization, facilitating access to various molecular frameworks that are not otherwise readily accessible.
We report an unprecedented reactivity of proline and demonstrate, for the first time, its ability to function as a reducing agent in the dehydroxylation process of α-ketols. The synthetic advantage of our method is exemplified by the simple and safe setup. The developed metal-free and open-flask operation generally results in good yields, minimal formation of side products, and allows the challenging reduction of hydroxy-ketones without affecting other functional groups.
The mechanism of discovered reaction will be unfolded,1 and findings will be projected on future utilization in organic synthesis.
1. Mostinski, Y.; Lankri, D.; Konovalov, Y.; Nataf, R.; Tsvelikhovsky, D. Chem. Sci. 2019, DOI: 10.1039/C9SC02543J.
Powered by Eventact EMS