
Invited
Proton-Coupled Electron Transfer (PCET) reactivity of molecules and nanoscale interfaces
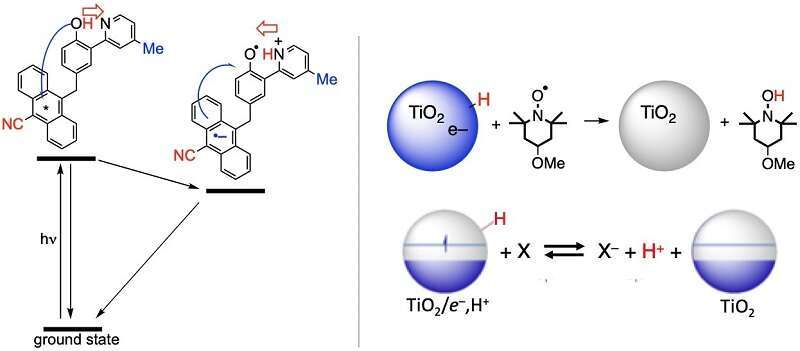
Chemical oxidation and reduction (redox) reactions are typically described in terms of the transfer of electrons, but in many cases protons play an equally important role. Such proton-coupled electron transfer (PCET) reactions are central to many chemical processes, from the biological ‘electron transport chain’ to catalysis in fuel cells. The movements of a proton and an electron are often coupled thermodynamically, and kinetically they often occur in a single chemical step. For example, in the molecular anthracene-phenol-pyridine triad shown below, excitation of the anthracene leads to two consecutive PCET steps, and the second one has been shown to be in the Marcus inverted region, the first such example for PCET.1 In materials chemistry, PCET is much less frequently invoked but we argue that it is very common in reactions at solid/solution interfaces.2 This presentation will describe such reactions of colloidal aqueous TiO2 nanoparticles (see below), NiO electrodes, and other systems. The coupling of protons of electrons in interfacial reactivity compels a re-examination of the description of these pervasive reactions.2
-
“Concerted Proton-Electron Transfer Reactions in the Marcus Inverted Region” Parada, G.; Goldsmith, Z.; Kolmar S.; Rimgard, B.P.; Mercado, B.Q.; Hammarström, L.; Hammes-Schiffer, S.; Mayer, J.M. Science 2019, 364, 471-475.
-
“Manifesto on the Thermochemistry of Nanoscale Redox Reactions for Energy Conversion” Peper, J. L.; Mayer, J. M. ACS Energy Lett. 2019, 4, 866−872.
Powered by Eventact EMS