
Surprisingly simple catalytic access to stereodefined fully substituted Aldehyde Enolates
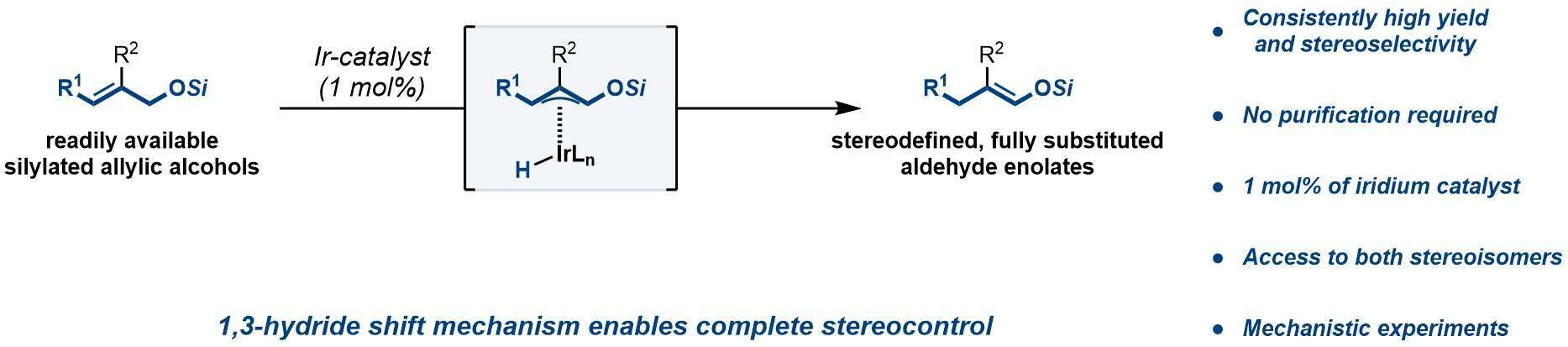
Fully substituted enolates are intuitive precursors to quaternary stereocenter-containing carbonyl compounds. However, control of the double bond stereochemistry for such enolates, which dictates the stereoselectivity of subsequent transformations, is a largely unsolved problem, thus limiting their use in stereoselective synthesis. Herein, we disclose a stereoselective approach towards fully substituted aldehyde enolates based on catalytic alkene isomerization. In the presence of 1.0 mol% of an Ir-based alkene isomerization catalyst, simple allylic silyl ethers are transformed into valuable, fully substituted aldehyde-derived silyl enol ethers. Mechanistically, the isomerization likely proceeds through a pi-allyliridium intermediate, which enables complete stereocontrol over enolate stereochemistry, regardless of the steric properties of the two alpha substituents. Importantly, by judicious choice of substrate, either of the two possible stereoisomers of a given enolate is accessible with high stereoselectivity. We further showcase the utility of the method through a one-pot isomerization-Mukaiyama aldol sequence, which affords aldol products bearing adjacent quaternary and tertiary stereocenters with complete diastereoselectivity. Finally, the dramatic effect of subtle changes in the metal/ligand stoichiometric ratio on the behavior of the catalytic system will be discussed and rationalized.
Powered by Eventact EMS