
Regio- and diastereoselective copper-catalyzed carbometalation of cyclopropenylsilanes
Polysubstituted stereodefined cyclopropanes are versatile building blocks in organic chemistry[1] as well as common motive in natural products and biologically active molecules[2]. Nevertheless, only a few methods exist to synthesize these reactive molecules in a stereodefined manner. In past two decades carbometallation of cyclopropenes was demonstrated as a powerful method in this context[3]. Large variety of enantioenriched cyclopropenation methods reported[4-7], allows to develop diastereoselctive carbomatallations, and access a broad scope of stereodefined cyclopropanes. The synthesis of fully substituted cyclopropanes in stereoselective manner was never reported. We suggest the diastereoselctive synthesis of the latter by copper catalyzed carbometallation of cyclopropenylsilanes. This method was chosen as the precursors are easily accessible from enantioenriched cyclopropenes, and presence of silicon atom will dictate the regioselectivity of the addition reaction on the reactive double bond of the cyclopropene. Quenching cyclopropyl metal intermediate with suitable electrophiles will allow to reach the desired fully substituted cyclopropanes.
[1] I. Marek, A. Masarwa, P. O. Delaye and M. Leibeling, Angewandte Chemie International Edition, 2015, 54, 414-429. [2] D. Y. K. Chen, R. H. Pouwer and J.-A. Richard, Chemical Society Reviews, 2012, 41, 4631-4642. [3] D. S. Müller and I. Marek, Chemical Society Reviews, 2016, 45, 4552-4566. [4] Y. Lou, M. Horikawa, R. A. Kloster, N. A. Hawryluk and E. J. Corey, Journal of the American Chemical Society, 2004, 126, 8916-8918. [5] H. M. L. Davies and G. H. Lee, Organic Letters, 2004, 6, 1233-1236. [6] M. Uehara, H. Suematsu, Y. Yasutomi and T. Katsuki, Journal of the American Chemical Society, 2011, 133, 170-171. [7] D. T. Boruta, O. Dmitrenko, G. P. A. Yap and J. M. Fox, Chemical Science, 2012, 3, 1589-1593. 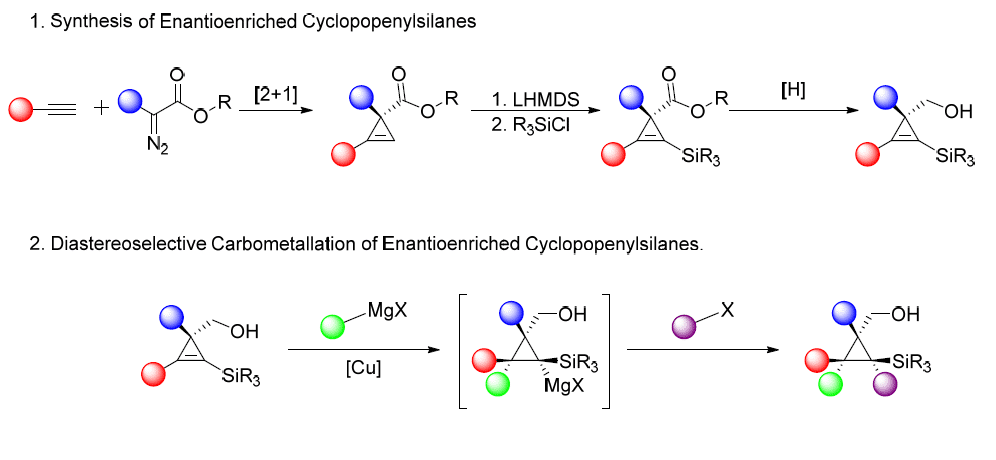
Powered by Eventact EMS