
Site-selective acylation of amphiphilic substrates with nucleophilic organocatalysts based on multibranched/dendritic design
While the field of organocatalysis underwent fast development in the past decades, many organocatalytic systems still suffer from low activity or selectivity. The low activity can be sometimes counterbalanced by increasing the reaction time or the catalyst loading. The impaired selectivity is, however, irreparable.
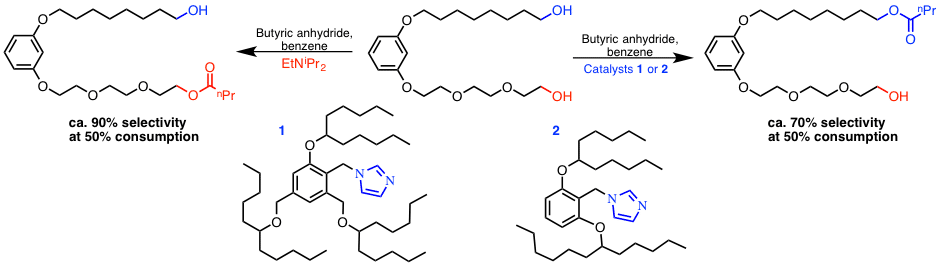
A new design of organocatalysts, embracing a nucleophilic active site surrounded by long linear or branched tails, potentially controlling the access of substrate molecules to the catalytic core, was explored in our group with the aim of achieving site-selectivity in chemical transformations of diol or polyol substrates. Thus, in an acylation of model amphiphilic diol substrate with butyric anhydride, the use of a nucleophilic catalyst, particularly branched/dendritic catalyst with the imidazole site in the core, enabled a remarkable shift of the site-selectivity from a polar alcohol site, preferred in background non-catalyzed or base-promoted reactions, to an apolar site (Scheme 1). Elucidation of the reasons of the site-selectivity, observed in each case, was achieved by performing a complimentary set of substrate-competitive acylations of polar and apolar mono-alcohol substrates.
A successful implementation of the insights, deduced from these results, to additional catalytic systems and substrates, including a natural macrolide amphiphilic antibiotic, will be presented at the talk.
Scheme 1
Powered by Eventact EMS