
Ab initio calculations of the rotational spectrum of hydroperoxy-acetaldehyde and ethylperoxide
2Division of Atomic and Molecular Physics, Harvard Smithsonian Center for Astrophysics, Cambridge, MA, USA
3Chemistry, IAEC, Tel Aviv, Israel
Peroxides are of great interest in atmospheric and combustion chemistry. They often play a major role in the radical portions of reaction networks under extreme conditions.
Peroxides pose challenges both experimentally and theoretically – their numerous conformers, instability and unusual structure make them difficult to detect, and complex behaviors such as tunneling complicate the theoretical structural calculations.
Peroxides produced from ozonolysis of alkenes are of particular importance. The simplest alkene, ethylene, is released into the atmosphere by plants and thus may react with atmospheric ozone to form a 5-membered ring, c-C2 H4 O3. This, in turn, dissociates to form CH2=O & CH2=OO, which subsequently fragments to H, OH, and CO. A different possible path involves ring opening followed by H atom migration to produce CHO-CH2-OOH, hydroperoxy-acetaldehyde, a keto-hydroperoxide (KHP). This species was recently reported to be initially observed in a stirred jet system using VUV photoionization mass spectrometry [1]. We intend to detect KHP by chirped-pulse microwave spectroscopy, using high resolution microwave spectroscopy and a setup similar to Womack et. al. [2]. Ethylperoxide is another important species that is expected to take a role in this scheme of reactions.
We predicted the rotational spectrum of the lowest lying conformers of KHP and ethylperoxide with high accuracy ab initio calculations. The geometry optimization was performed at the all-electron CCSD(T)/cc-pCVQZ level of theory, and anharmonic effects were incorporated through VPT2 calculations using the frozen core CCSD(T)/ANO0 level of theory. This structure was found to have a hydrogen bond between the peroxy H atom and the carbonyl O atom, stabilizing it substantially compared to other conformers. MP2/ANO0 calculations showed that this conformer’s energy is the lowest by at least 400 cm^-1 in comparison to other possible conformers. No other stable conformers of ethylperoxide were found.
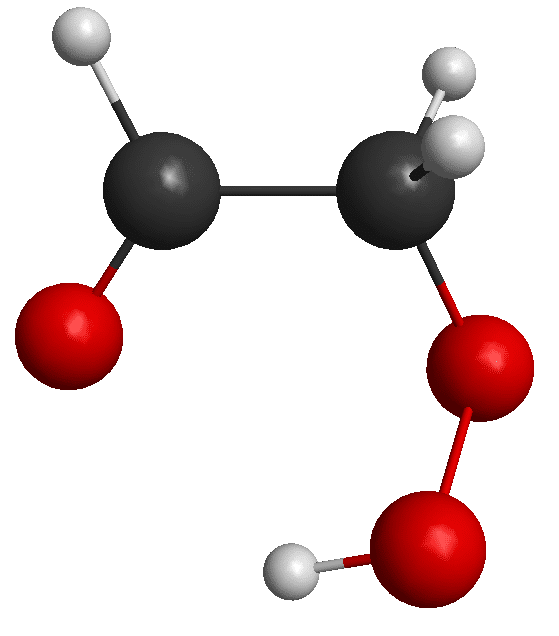
[1] Rousso et. al., J. Phys. Chem. A, 122, 8674-8685, 2018.[2] Womack et. al., Sci. Adv. 1 (2), e1400105, 2015.
Powered by Eventact EMS