
Oxidative transformations of enolates and heteroarenes enabled by palladium and nickel catalysis
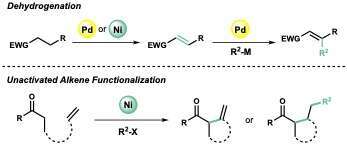
The use of Pd and Ni catalysis in conjunction with unconventional allyl or aryl oxidants has enabled the development of novel dehydrogenation technologies that allow for the one-step introduction of a double bond adjacent to carbonyls and heteroarenes. This catalytic manifold was further leveraged for the development of 1) an oxidative β-functionalization of enones, which was used in the total synthesis of xylogranatopyridine B, a limonoid alkaloid natural product, as well as 2) an oxidative cycloalkenylation of unstabilized ketone enolates, which provided access to a diverse range of carbocyclic architectures using abundantly available ketones and unactivated alkenes as reaction partners. Mechanistic insights into the cycloalkenylation process has also led to the development of a Ni-catalyzed difunctionalization of unactivated alkenes with unstabilized enolates using a diverse range of aryl, heteroaryl, alkenyl, and amino electrophiles.
Powered by Eventact EMS