
Catalytic selective N-Oxidation of loratadine analogs
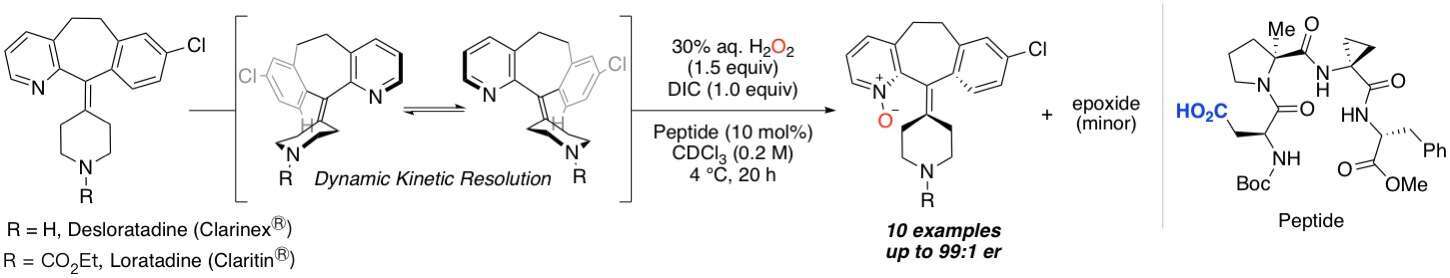
Analogs of Claritin® (loratadine) and Clarinex® (desloratadine), some of the top selling over-the-counter antihistamines, have been enantio- and site-selectively N-oxidized. This catalytic system utilizes aspartic acid embedded peptides that shuttle between free acid and peracid forms to generate N-oxide containing products, which possess a relatively unique type of helical chirality. While the (des)loratadine analogs contain an alkene and pyridine, both of which are susceptible to oxidation, the reaction conditions favor formation of the N-oxide, achieving up to 99:1 er. The conformational dynamics of the loratadine starting material and enantiomeric stability of the N-oxide products are being investigated both experimentally and computationally with the aid of crystallographic data. Substrate-catalyst binding interactions have been highlighted in the direct correlation between er (ΔΔG‡) and the Hammett coefficient of a p-substituted aryl urea directing group appended to the desloratadine core. Furthermore, rigidifying the flexible structure of (des)loratadine likely affects target binding, thus, each enantiomer of a representative N-oxide product is being tested for biological activity.
Powered by Eventact EMS