
Reversible system for selective oxidation in acidic media
H5PV2Mo10O40 (1) polyoxometalate (POM) are electron transfer (ET) and electron transfer−oxygen transfer (ET−OT) catalysts for selective oxidation of hydrocarbons. In many organic solvents, the scope of these reactions was limited by the oxidation potential of 1, which is ca. 0.4−0.45 V1. Recently, however, Neumann found that in 50% H2SO4 the reduction potential of 1 was increased to 1.1 V, and as a result, able to oxidize C-H bonds of toluene2 and benzene3 to benzaldehyde and phenol respectively. To better understand the nature of 1 in H2SO4, we (in collaboration with the Neumann group) have been investigating the solution-state chemistry of 1 as a function of H2SO4 concentration. Recent findings suggest that 1 rearranges in 50% H2SO4 so as to release V(V) (VO2+ in the presence of H2SO4)4, and this species is responsible for the strongly oxidizing properties of the system while the selectivity is governed by in-situ formed [(Mo2O5)2+]. In cyclic voltammograms (CVs) of 1 in 50% H2SO4, for example, an electrochemically reversible redox couple is observed at 1.2 V, effectively identical to those obtained when NaVO3 was dissolved in 50% H2SO4. Next, the release of PO43- and vanadate (as VO2+) as a function of H2SO4 concentration were confirmed by 31P- and 51V-NMR respectively. Frozen state EPR spectra of reduced oxidant solution in 50% H2SO4 confirmed the presence of isolated vanadyl cation (VO2+). Further, substrate-oxidation studies suggested that the actual reactive oxidant is the dioxovanadium cation (VO2+) and the selectivity is enhanced by molybdate cation [(Mo2O5)2+] which was isolated from the solution of 1 in 50% H2SO4 and the structure was solved by single-crystal XRD.
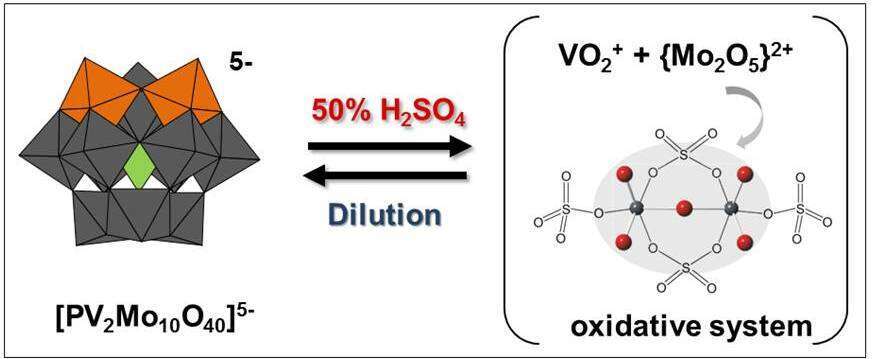
Figure: Reversible System for Selective Oxidation
References:
- Neumann, R. Inorg. Chem., 2010, 49, 3594−3601
- Sarma B. B., Efremenko I., and Neumann R., J. Am. Chem. Soc., 2015, 137, 5916–5922
- Sarma B. B., Carmieli R., Collauto A., Efremenko I., Martin M. L. Jan and Neumann R., ACS Catal. 2016, 6, 6403−6407
- Freund M. S. and Lewis N.S., Inorg. Chem. 1994, 33, 1638-1643
Powered by Eventact EMS