
Visible-light water oxidation by polyoxometalate complexed γ-FeOOH nanocrystals
Polyoxometalate (POM) cluster-anions can now be covalently attached to inherently stable metal-oxide nanocrystals in water. The POM-complexed metal-oxide cores, comprising hundreds to thousands of metal atoms, range from several to tens of nm in diameter and can harness solar light to perform water-splitting.[1] Using this methodology, we recently investigated visible-light driven water oxidation by hematite α-Fe2O3 nanocrystals, that bridge a conceptual divide between molecular complexes of iron and solid-state hematite photoanodes.[1bc] The aqueous solubility and remarkable stability of polyoxometalate- (POM-) complexed 275 iron-atom hematite cores made it possible to investigate the reaction using the versatile toolbox of solution-state methods typically reserved for molecular catalysis.[1c] The data revealed a unique radical-chain mechanism, understood as a general consequence of fundamental differences between reactions of solid-state metal oxides and freely diffusing "fragments" of the same material. Building on those findings, we now investigate the mechanism of visible-light water oxidation by POM-complexed iron-oxy-hydroxide (γ-FeOOH) (see Figure 1). The kinetic data again reveal a radical-chain mechanism, confirming this pathway as a general property of electron-capture controlled visible-light driven homogeneous-phase catalysis of water oxidation by metal nanocrystals.
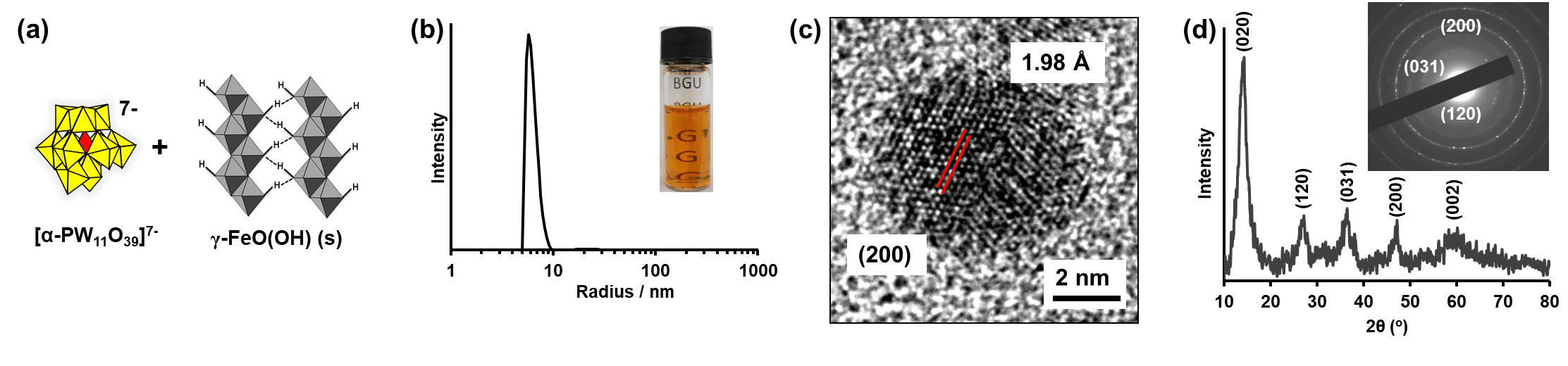
Figure 1. Preparation of POM complexed γ-FeOOH nanocrystals.
[1] (a) M. Raula, G. Gan Or, M. Saganovich, O. Zeiri, Y. Wang, M. R. Chierotti, R. Gobetto, I. A. Weinstock, Angew. Chem. Int. Ed., 2015, 54, 12416-12421; (b) B. Chakraborty, G. Gan Or, M. Raula, E. Gadot, I. A. Weinstock, Nat. Comm., 2018, 9: 4896; (c) B. Chakraborty, G. Gan Or, Y. Duan, M. Raula, I. A. Weinstock, Angew. Chem. Int. Ed., 2019, 58, 6584-6589.
Powered by Eventact EMS