
A mechanism of nonclassical protein crystallization
2Chemical Research Support, Weizmann Institute of Science, Rehovot, Israel
Protein crystallization is important in structural biology, disease control, and pharmaceuticals. It has been recently recognized that nonclassical crystallization, involving initial formation of amorphous precursor phase, often occurs in protein, organic, and inorganic crystallization processes. Consequently, two-step nucleation theory was put forward, postulating initial formation of low density solvated amorphous aggregates that densify and lead to nucleation. This view differs substantially from classical nucleation theory, although a classical pathway has been recently directly observed. However, a molecular mechanism of nonclassical protein crystallization has not been elucidated. Herein we present a study of ferritin crystallization using cryo-STEM tomography1 for 3D quantitative density and order analysis of a nonclassical crystallization mechanism.2
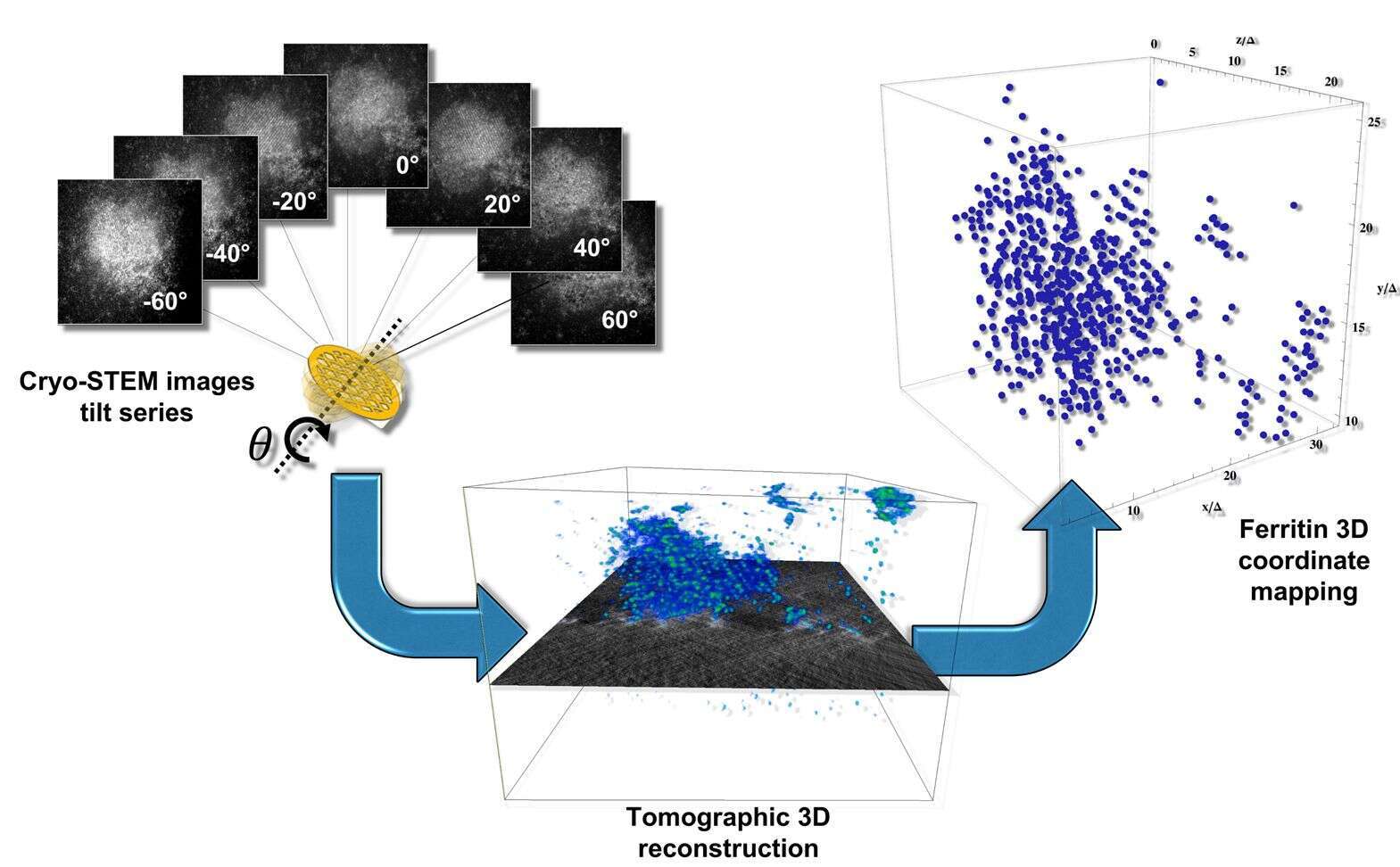
Figure 2. Methodological approach for the three-dimensional characterization of ferritin crystallisation. Tilt series of images are recorded in STEM dark-field mode, highlighting the Fe-rich core of the ferritin molecules. Subsequent tomographic reconstruction leads to a three-dimensional volume of intensities. Volume peak refinement yields the center-of-mass coordinates of each ferritin molecule in three dimensions.
References
1. Wolf, S. G., Houben, L. & Elbaum, M. Nat. Methods 11, 423 (2014).
2. Houben, L., Weissman, H., Wolf, S. G. & Rybtchinski, B. Nature (2019), Submitted.
Powered by Eventact EMS