
Unprecedented selectivity of ruthenium iodide benzylidenes towards unhindered olefins and its unpredictable consequences
Sulfur-chelated Ru benzylidenes facilitate anionic ligand exchange due to the enhanced trans influence of the strongly sigma donating NHC ligand; by this fashion Ru-S-I could be readily obtained (Figure 1). While Ru-S-I was extremely efficient in ring closing metathesis (RCM) reactions, it was completely inert towards ring opening metathesis polymerization (ROMP).1 This never before seen behavior led us to investigate the reactivity of Ru-S-I towards several other reactive substrates. Indeed, all ROMP monomers were completely inert. Moreover, when DEDAM was combined with norbornene derivatives, only RCM occurred. However, a most intriguing outcome was obtained when cyclooctenes were mixed with DEDAM: the complete consumption of all metathesis substrates. When the DEDAM/COE ratio is large, 1,9-decadiene was the main product; while the opposite ratio lead to oligomers reminiscent of an ADMET mechanism. We propose that while the alkylidene complex is too bulky to coordinate a non-terminal olefin, the methylidene intermediate formed by the RCM reaction is just the right size to react with cyclooctene (but not the norbornenes). This unexpected behavior allows the ring-closing metathesis of 1,9-undecadiene to produce strained cis-cyclooctene with good selectivity. Moreover, a mixture of the diiodo complex with polybutadiene and a small amount of a terminal olefin initiator catalyzes a depolymerization process yielding small cyclic molecules, including the highly strain 1,5-cyclooctadiene. Perhaps the most fascinating result is that when mixing neat DCPD (the most reactive and useful ROMP monomer) with Ru-S-I, the liquid mixture remains unaffected, highlighting the complexes’ impressive latency for this important industrial monomer. To our great satisfaction, addition of (Bu)4NCl even after one week standing, immediately activated the complex by a facile exchange of the anionic ligands forming Ru-S-Cl in situ and polymerizing the mixture!
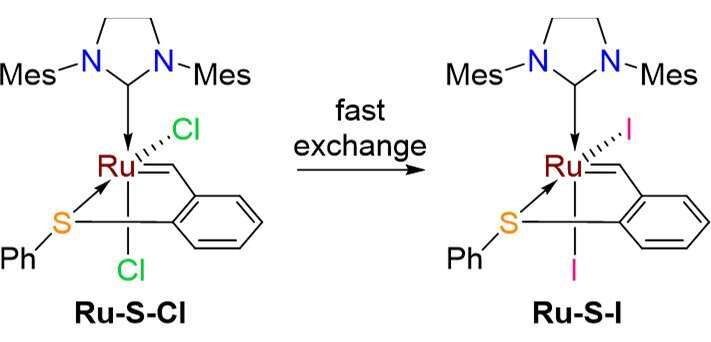
Figure 1. anionic ligand exchange
[1] Ivry, E., Nechmad, N.B., Baranov, M., Goldberg, I. and Lemcoff, N.G., Inorg. Chem., 2018, 57, 15592-15599.
[2] Noy B. Nechmad, Ravindra Phatake, Elisa Ivry, Albert Poater, and N. Gabriel Lemcoff, Submitted.
Powered by Eventact EMS