
A stereoselective approach towards trisubstituted vinylborononates through Iridium Catalyzed Alkene Isomerization
Schulich Faculty of Chemistry, Technion – Israel Institute of Technology, Haïfa, Israel
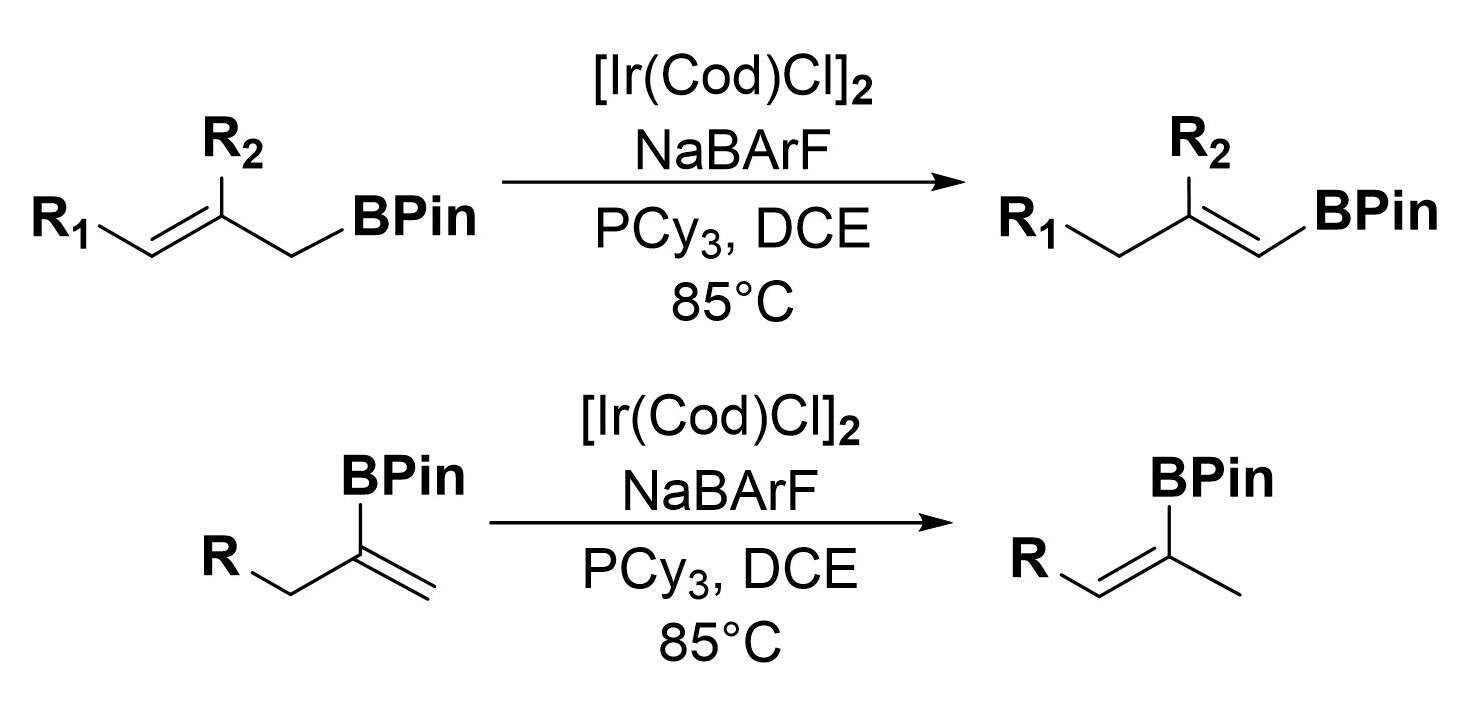
The synthesis of vinylboron species through hydroboration is often limited by issues of regio- and stereoselectivity. In cases where hydroboration is not suitable, alkene isomerization is an attractive strategy to bridge between easily accessible vinylboron species and their more substituted counterparts.
Using a cationic Iridium-based system, the above strategy was successfully applied to give both good yield, high stereo- and regioselectivity from simpler regioisomers by relying on the inherent selectivity of the 1,3-hydride shift mechanism. Different positions of the initial alkene and the substitution pattern of the starting material allow the control of the structure of the obtained product.
Powered by Eventact EMS