
Linking aqueous trehalose glass-to-liquid transition with hydrogen bonding properties
2Department of Mechanical Engineering and Materials Science,, Duke University, Durham, NC, USA
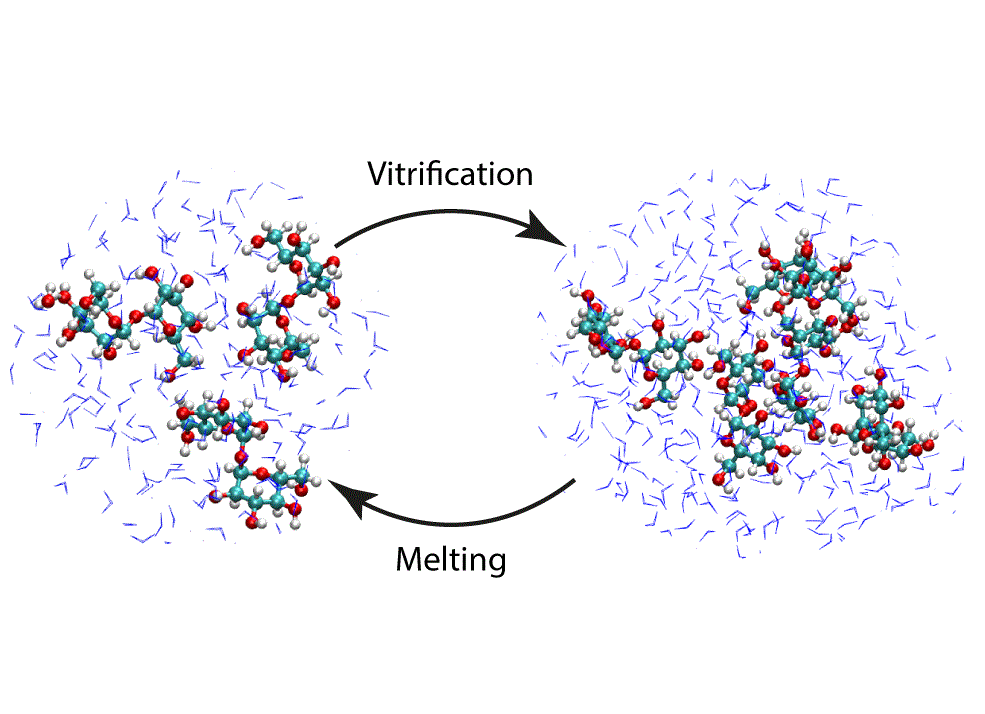
Molecular mixtures that form glassy materials play an important role in the survivability of many biological life forms, and has technological implications, from desiccation protection to cryopreservation. An important example is the disaccharide trehalose, which is known for its exceptional ability to thermodynamically stabilize biomacromolecules such as proteins, lipids, and DNA in the biologically active state. Of special interest is the transition from aqueous solution to the dense and highly concentrated glassy state of trehalose that has been implicated in bioadaptation of different organisms toward desiccation stress. The glassy state is a metastable form of a liquid that forms when cooled below its melting temperature until at the glass transition temperature, the viscosity is large enough that relaxation is unattainable on experimental timescales. The link between the stabilization effect and the glassy state properties has not yet been resolved. A prominent property of glass forming sugars is the complex hydrogen bonding network that constitutes both the sugar and water collectively. Here we use molecular dynamics simulations to decipher the link between structure and properties of trehalose-water mixtures in both liquid and the supercooled glassy state. We develop and employ a methodology that allows us to analyze the thermodynamics of hydrogen bonds in simulations at different water contents and temperatures. Remarkably, this analysis is able to link the liquid to glass transition with changes in hydrogen bond characteristics as it is sensitive to the coupling of hydrogen bonded pairs to their surrounding upon transition. As the link between hydrogen bonding and the glass transition is not evident in other conventional methods, our findings should help resolve the properties of the glass and the mechanisms of its formation in the presence of added macromolecules.
Powered by Eventact EMS