
Design and synthesis of higherorder cyclodextrin architectures: Targeted system performance
Cyclodextrin is an important host for a variety of applications including drug delivery, sensing and catalysis. It has been extensively studied as a supramolecular scaffold that bind molecules in aqueous solutions, through hydrophobic binding, intermolecular hydrogen bonding, and/or through the displacement of “high energy” water.1 Many non-covalent interactions in cyclodextrin complexes have relevance in research areas that depends on such interactions, including in enzyme-substrate binding and in the development of new pharmaceutical agents.2 Monomeric cyclodextrins have provided an effective framework to investigate these interactions,3 and have led to beneficial outcomes in a variety of applications.4 Previously the Levine group has reported cyclodextrin-promoted analyte detection;5 binding of squaraine in cyclodextrin dimers;6 and the synthesis and applications of cyclodextrin MOFs.7 Motivated by our previous research, herein we plan to investigate higher order cyclodextrins, including both dimers and trimers (Fig.1). By investigating the structures of these compounds using experimental and computational approaches, and their ability to bind guests in and around their cyclodextrin cavities, we will obtain a detailed understanding of how the structures of these compounds inform and determine their supramolecular properties.
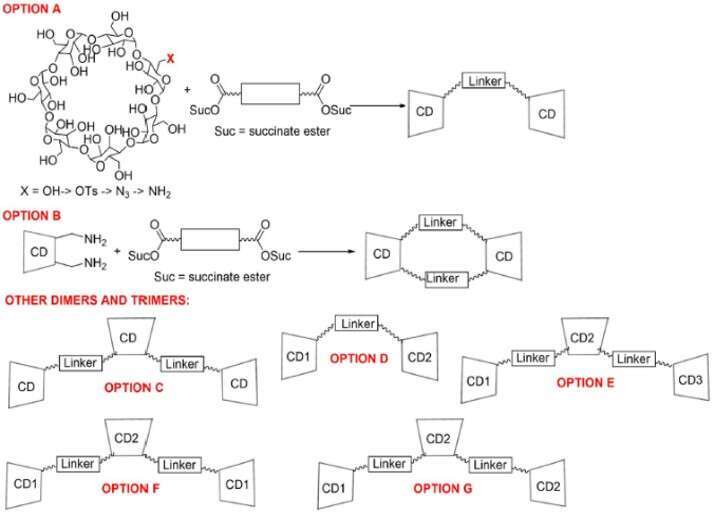
Fig 1: Schematic representation of the general design of cyclodextrin dimers and trimers to be synthesized in this study.
References
1. (a). Schneider, H.-J., Acc. Chem. Res. 2015, 48, 1815-1822. (b). Breslow, R.; Dong, S. D., Chem. Rev. 1998, 98, 1997-2011.
2. Kellett, A.; Molphy, Z.; Slator, C.; McKee, V.; Farrell, N. P., Chem. Soc. Rev. 2019, 48, 971-988.
3. Kundu, M.; Rahaman, H.; Roy, M. N., Spectrochim. Acta A, 2019, 218, 9-14.
4. Fernandez, M. A.; Silva, O. F.; Vico, R. V.; de Rossi, R. H., Carbohydrate Res., 2019, 480, 12-34.
5. DiScenza, D. J.; Levine, M., New J. Chem. 2016, 40, 789-793.
6. Chaudhuri, S.; Verderame, M.; Mako, T. L.; Bandara, Y. M. N. D. Y.; Fernando, A. I.; Levine, M., Eur. J. Org. Chem. 2018, 2018, 1964-1974.
7. Jones, D. R.; DiScenza, D. J.; Mako, T. L.; Levine, M., J. Chem. Educ. 2018, 95, 1636-1641.
Powered by Eventact EMS